The Correlation of P53 and MSI Immune Markers in Gastric Adenocarcinoma
DOI:
https://doi.org/10.32007/jfacmedbagdad.6622290Keywords:
Gastric adenocarcinoma, molecular classification; , immunohistochemistry;, P53; , microsatellite instabilityAbstract
Background: The known risk factors for gastric adenocarcinoma are chromosomal instability, TP53 mutations, aneuploidy, translocations, proto-oncogenes, and tumor suppressor gene changes. Microsatellite instability (MSI) affects DNA replication accuracy and is detected by the heterodimeric protein complex hMSH2/hMSH6, which recruits hMLH1 and hPMS2 for re-synthesis. MSI can cause sporadic gastric cancer and Lynch syndrome.
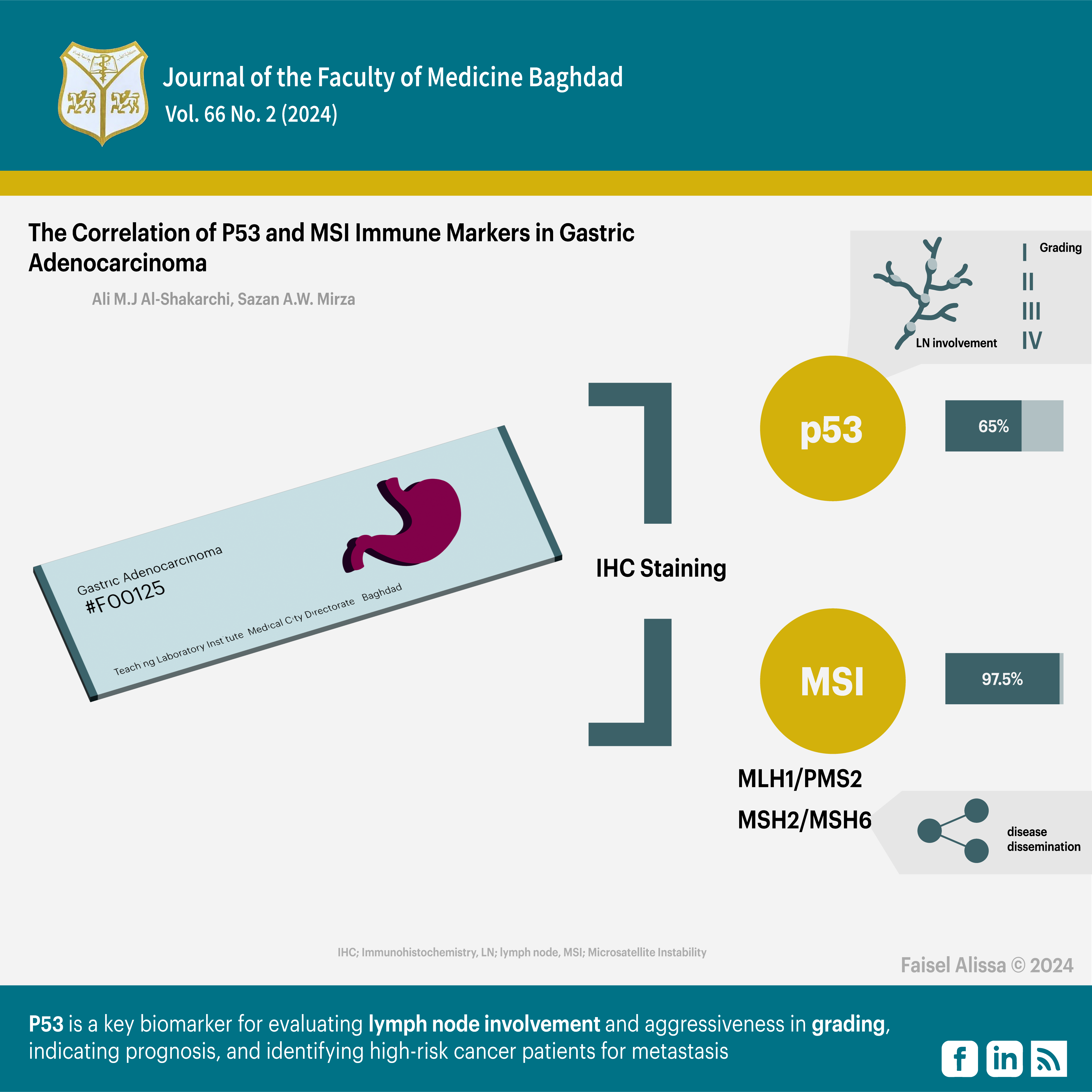
Objectives: To examine the relationship between P53 and MSI immune markers expression with the clinicopathological parameters of gastric adenocarcinoma by using immunohistochemistry.
Methods: The study examined 40 formalin-fixed, paraffin-embedded gastric adenocarcinoma tissue blocks. The samples were retrieved from archived materials in the histopathology department of the Gastroenterology and Hepatology Teaching Hospital, Teaching Laboratory Institute, and some private laboratories in Baghdad, Iraq. The samples were taken from patients between 2020 and 2023, while their retrieval spanned from October 2022 to October 2023 for the sake of examining primary cases, surgical tissues, and available clinicopathological data. The immunohistochemical (IHC) expression was assessed using a scoring system. Data were analyzed using SPSS, Chi-square, and Fisher's exact tests, with a 95% confidence level and a 0.05 P-value or less considered significant.
Results: IHC staining for P53 was positive in 65% of the samples, while MSI findings were positive in 97.5% of the samples. The MLH1/PMS2 heterodimeric couple showed 32.5% positive results, while the MSH2/MSH6 heterodimeric couple showed 87.5% positive results. P53 stain was significantly correlated with lymph node involvement and grade, but not with the other parameters. No significant association was found between MSI markers and the studied parameters. There was no significant association between MSI heterodimeric couple (MLH1/PMS2) and the clinicopathological parameters, but there was a significant association between MSI heterodimeric couple (MSH2/MSH6) markers and metastasis only.
Conclusion: P53 is a key biomarker for evaluating lymph node involvement and aggressiveness in grading, indicating prognosis, and identifying high-risk cancer patients for metastasis.
Received: Jan. 2024
Revised: Mar. 2024
Accepted: April, 2024
Published: July. 2024
Downloads
References
1. Ilic M, Ilic I. Epidemiology of stomach cancer. World journal of gastroenterology. 2022;28(12):1187. https://doi.org/10.3748/wjg.v28.i12.1187
2. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. International journal of cancer. 2021;149(4):778-89. https://doi.org/10.1002/ijc.33588
3. Mwafaq RK, Abbas AK, Abdullah LAH. Evaluation of PARP-1 by imm unohistochemistry in a sample of Iraqi patients with gastric cancer. Iraqi Journal of Science. 2022:467-79. https://doi.org/10.24996/ijs.2022.63.2.5
4. Ashour HJ, Al-Bakri NA, Abdul Ghafour KH. The Immunohistochemical Assessment of Muc5ac in Patients with Gastric Carcinoma (Gc) in Iraq. Iraqi Journal of Science. 2019:460-8. https://www.ijs.uobaghdad.edu.iq/index.php/eijs/article/view/683
bservatory gc. global cancer observatory 2023 [Available from: https://gco.iarc.fr/today].
6. Toh JW, Wilson RB. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. International journal of molecular sciences. 2020 Sep 3;21(17):6451. https://doi.org/10.3390/ijms21176451
7. Torbenson M, Ng I, Park Y, Roncalli M, Sakamato M. WHO Classification of Tumours. Digestive System Tumours. Lyon, France: International Agency for Research on Cancer. 2019:229-39. https://dokumen.pub/who-classification-of-tumours-5th-edition-digestive-system-tumours-1-5nbsped-9789283244998.html
8. Lauwers G, Kumarasinghe P, Cytopath D. Gastric cancer: Pathology and molecular pathogenesis. UpToDate, UptoDate, Waltham, MA. 2020. https://www.medilib.ir/uptodate/show/119517
9. Nishizawa T, Nakano K, Harada A, Kakiuchi M, Funahashi S-I, Suzuki M, et al. DGC-specific RHOA mutations maintained cancer cell survival and promoted cell migration via ROCK inactivation. Oncotarget. 2018;9(33):23198. https://doi.org/10.18632/oncotarget.25269
10. Nakayama I, Shinozaki E, Sakata S, Yamamoto N, Fujisaki J, Muramatsu Y, et al. Enrichment of CLDN18‐ARHGAP fusion gene in gastric cancers in young adults. Cancer Science. 2019;110(4):1352-63. https://doi.org/10.1111/cas.13967
11. Al-Badri BA, Ali GQ. P53 Expression in Gastric Dysplasia and carcinoma in Erbil city. Journal of the Faculty of Medicine Baghdad. 2011;53(2):190-3. https://doi.org/10.32007/jfacmedbagdad.532869
12. Habib M. The possible role of EBV in carcinogenesis of colorectal carcinoma. Fac Med Baghdad. 2010;52:172-4. https://doi.org/10.32007/jfacmedbagdad.5221016
13. Yang J, Liu Z, Zeng B, Hu G, Gan R. Epstein–Barr virus-associated gastric cancer: a distinct subtype. Cancer letters. 2020 Dec 28;495:191-9. https://doi.org/10.1016/j.canlet.2020.
14. Ramos MFKP, Pereira MA, de Mello ES, dos Santos Cirqueira C, Zilberstein B, Alves VAF, et al. Gastric cancer molecular classification based on immunohistochemistry and in situ hybridization: Analysis in western patients after curative-intent surgery. World Journal of Clinical Oncology. 2021;12(8):688. https://doi.org/10.5306/wjco.v12.i8.688
15. Ratti M, Lampis A, Hahne JC, Passalacqua R, Valeri N. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cellular and Molecular Life Sciences. 2018;75:4151-62. https://doi.org/10.1007/s00018-018-2906-9
16. Grosser B, Kohlruss M, Slotta-Huspenina J, Jesinghaus M, Pfarr N, Steiger K, et al. Impact of tumor localization and molecular subtypes on the prognostic and predictive significance of p53 expression in gastric cancer. Cancers. 2020 Jun 25;12(6):1689. https://doi.org/10.3390/cancers12061689
17. Hwang HJ, Nam SK, Park H, Park Y, Koh J, Na HY, et al. Prediction of TP53 mutations by p53 immunohistochemistry and their prognostic significance in gastric cancer. Journal of pathology and translational medicine. 2020 Jul 1;54(5):378-86. https://doi.org/10.4132/jptm.2020.06.01
18. Kim KW, Kim N, Choi Y, Kim WS, Yoon H, Shin CM, et al. Different effects of p53 protein overexpression on the survival of gastric cancer patients according to Lauren histologic classification: a retrospective study. Gastric Cancer. 2021 Jul;24:844-57. https://doi.org/10.1007/s10120-021-01163-y
19. Zhang X, Wang M, Wang Y, Cheng X, Jiang Y, Xiao H. Clinicopathologic significance of Her-2 and P53 expressions in gastric cancer. Asian Journal of Surgery. 2023;46(1):526-31. https://doi.org/10.1016/j.asjsur.2022.06.039
20. Karpińska-Kaczmarczyk K, Lewandowska M, Ławniczak M, Białek A, Urasińska E. Expression of mismatch repair proteins in early and advanced gastric cancer in Poland. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2016;22:2886. https://doi.org/10.12659/MSM.897150
21. Hanon BM, Mohammad NAA-M, Mahmood AS. The Correlation Between Microsatellite Instability and the Features of Sporadic Colorectal Cancer in Sample of Iraqi Patients. Journal: Journal Of Advances In Biotechnology.4(1). https://doi.org/110.24297/jbt.v4i1.1632
22. Tanabe H, Mizukami Y, Takei H, Tamamura N, Omura Y, Kobayashi Y, et al. Clinicopathological characteristics of Epstein–Barr virus and microsatellite instability subtypes of early gastric neoplasms classified by the Japanese and the World Health Organization criteria. The Journal of Pathology: Clinical Research. 2021;7(4):397-409. https://doi.org/10.1002/cjp2.209
23. Reitsam NG, Märkl B, Dintner S, Waidhauser J, Vlasenko D, Grosser B. Concurrent loss of MLH1, PMS2 and MSH6 immunoexpression in digestive system cancers indicating a widespread dysregulation in DNA repair processes. Frontiers in Oncology. 2022;12:1019798. https://doi.org/10.3389/fonc.2022.1019798
24. Evaristo G, Katz A, Ramírez-GarcíaLuna JL, Issac MS, Sangwan V, Thai D-V, et al. Relation between mismatch repair status, chemoresponse, survival and anatomic location in gastroesophageal adenocarcinoma. Canadian Journal of Surgery. 2023;66(1):E79. https://doi.org/10.1503/cjs.017021
25. Zhang L, Wang Y, Li Z, Lin D, Liu Y, Zhou L, et al. Clinicopathological features of tumor mutation burden, Epstein-Barr virus infection, microsatellite instability and PD-L1 status in Chinese patients with gastric cancer. Diagnostic Pathology. 2021;16(1):38. https://doi.org/10.1186/s13000-021-01099-y
26. Elrefaey HA, Mohamed DA, Al-Azim MAA-HA, El-Shorbagy SH, Helal DS. Microsatellite Instability and P53 Aberrant Expression in Gastric Adenocarcinoma: Immunohistochemical Assessment and Correlation with Clinico-pathological Characteristics. Journal of Advances in Medicine and Medical Research. 2023;35(14):21-42. https://doi.org/10.9734/jammr/2023/v35i145052
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Ali M.J Al-Shakarchi, Sazan A. M. Alatrooshi

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


