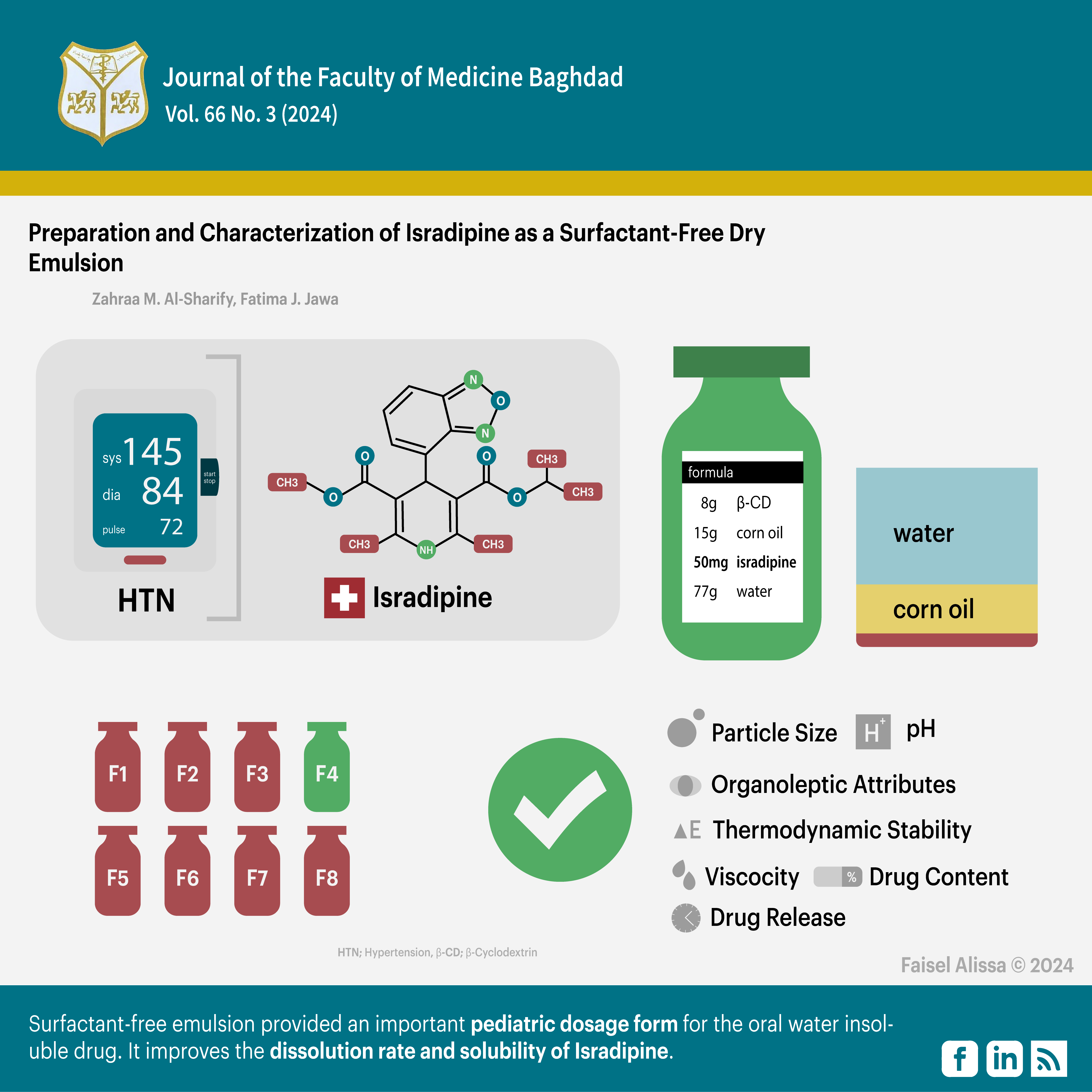
Preparation and Characterization of Isradipine as Surfactant-Free Dry Emulsion
DOI:
https://doi.org/10.32007/jfacmedbaghdad.6632262الكلمات المفتاحية:
Iraqi patients، NOAC، Quality of life، Satisfaction، Warfarinالملخص
Background: Oral anticoagulation medication, warfarin, and non-vitamin K antagonist oral anticoagulants (NOAC) may require long-term use which may affect patients’ satisfaction with their treatment and their quality of life (QOL).
Objective: To compare the quality of life and treatment satisfaction among groups of patients using different anticoagulant therapies (warfarin and NOAC).
Methods: A cross-sectional study was performed at Ibn Al-Bitar Hospital for cardiac surgery in Baghdad in the period from December 2022 to May 2023. The study population included a convenient sample of patients receiving either warfarin or non-vitamin k antagonist oral anticoagulants treatment. The Arabic version of the short form 12 (SF-12) questionnaire and the Anti-Coagulant Treatment Satisfaction Scale (ACTS) questionnaire were used to assess the quality of life and satisfaction with treatment respectively.
Results: The study included 181 patients in total. The mean physical and mental quality of life scores for study participants were 42.3±9.92 and 52.6±10.36 respectively. There was no significant difference in the QOL between patients taking warfarin and those on non-vitamin k antagonist oral anticoagulant treatment. The mean total satisfaction score was 65.4±6.73. Patients receiving non-vitamin k antagonist oral anticoagulants had significantly higher satisfaction compared to those receiving warfarin. The physical score correlated significantly with gender, educational level, employment status, number of chronic medications, and number of chronic diseases. The total satisfaction score correlated significantly with gender, number of chronic medications, number of side effects, and duration of anticoagulation. There was a significant correlation between the QOL and treatment satisfaction.
Conclusion: Treatment with non-vitamin K antagonist oral anticoagulants showed comparable QOL and higher treatment satisfaction than that of warfarin. Better treatment satisfaction can improve patients’ QOL which may ultimately enhance their adherence to treatment.
التنزيلات
المراجع
Subhi MD. Blood pressure profiles and hypertension in Iraqi primary school children. Saudi Med J. 2006;27(4):482-6. https://smj.org.sa/content/smj/27/4/482.full.pdf
Mohammed AM, Al-Rawi RA, Abdulmajeed BY, et al. The relationship between blood pressure and body mass index among primary-school children. Med J Babylon. 2022;19(3):482-487. https://doi.org/10.4103/MJBL.MJBL_91_22
Falkner B, Gidding SS, Baker-Smith CM, et al. Pediatric primary hypertension: An underrecognized condition: A scientific statement srom the American heart association. hypertension. 2023;80(6):e101-e111. https://doi.org/10.1161/HYP.0000000000000228
Akram NN, Abdullah WH, Ibrahim BA. Factors contribute to elevated blood pressure values in children with Type 1 diabetes mellitus: A Review. Med J Babylon. 2022;19(2):126-128. https://doi.org/10.4103/MJBL.MJBL_58_22
Abdoun DS, Al-Rawi RA, Abdulmajeed BY. Prevalence of Dyslipidemia and hypertension in Iraqi adolescents with type 1 diabetes mellitus. Al-Anbar medical J. 2022;18(2):77-81. https://doi.org/10.33091/amj.2022.176311
Laluna C.R., Pedregosa D.M., Abarca S.M., et al. A review of the safety and acceptability of commercially-available pediatric drug formulations. International J of research publication and reviews. 2023 ; 4(1): 440-455. https://doi.org/10.55248/gengpi.2023.4105
Golhen K, Buettcher M, Kost J, et al. Meeting challenges of pediatric drug delivery: The potential of orally fast disintegrating tablets for infants and children. Pharmaceutics. 2023; 15(4): 1-19. https://doi.org/10.3390/pharmaceutics15041033
Mahmood SZ., Yousif NZ, Salman ZD. Types of attractive dosage forms for primary school students and associated factors in Baghdad/Iraq. Al Mustansiriyah J of Pharmaceutical Sciences. 2020 ; 20(4): 13-22. https://doi.org/10.32947/ajps.v20i4.770
Karavasili C, Gkaragkounis A, Fatouros DG. Landscape of pediatric-friendly oral dosage forms and administration devices. Expert Opinion on Therapeutic Patents. 2021; 31(7): 663-685. https://doi.org/10.1080/13543776.2021.1893691
Guzmán E, Ortega F, Rubio RG. Pickering Emulsions: A novel tool for cosmetic formulators. Cosmetics. 2022 ; 9(4): 1-16. https://doi.org/10.3390/cosmetics9040068
Teixé-Roig J, Oms-Oliu G, Odriozola-Serrano I, et al. Emulsion-based delivery systems to enhance the functionality of bioactive compounds: Towards the use of ingredients from natural, sustainable sources. Foods. 2023 ; 12(7): 1-23. https://doi.org/10.3390/foods12071502
Peito S, Peixoto D, Ferreira-Faria I, et al. Nano- and microparticle-stabilized pickering emulsions designed for topical therapeutics and cosmetic applications. International J of pharmaceutics.. https://doi.org/10.1016/j.ijpharm.2022.121455
Zongguang T, Yanping H, Quangang Z, et al. Utility of Pickering emulsions in improved oral drug delivery. Drug discovery today. 2020 ; 00(00): 1-8. https://doi.org/10.1016/j.drudis.2020.09.012
Pawar AR, Belhekar SN, Mehetre JS. Enhancement of aqueous solubility and oral bioavailability of Bcs class II drug by dry emulsion. International J of pharmacognosy & Chinese medicine. 2021 ; 5(2): 11-12. https://doi.org/10.23880/ipcm-16000218
Mohammed NK, Tan CP, Manap YA. Spray drying for the encapsulation of oils-A review. Molecules. 2020 ; 25(3873): 1-16. https://doi.org/10.3390/molecules25173873
Raina R, Mahajan Z, Sharma A, et al. Hypertensive crisis in pediatric patients: An overview. Frontiers in Pediatrics.2020 Oct; 8: 1-17. https://doi.org/10.3389/fped.2020.588911
Hussien R, Ghareeb M. Formulation and characterization of Isradipine nanoparticle for dissolution enhancement. Iraqi J Pharm Sci. 2021; 30(1): 218-225. https://doi.org/10.31351/vol30iss1pp218-225
Al-Rubaye R, Al-Kinan KK. Preparation and characterization of Prednisolone Acetate microemulsion for ophthalmic use. J Fac Med Baghdad. 2023; 65(3): 205-211.
https://doi.org/10.32007/jfacmedbagdad.2045
Sadoon N, Ghareeb M. Formulation and characterization of isradipine as oral nanoemulsion. Iraqi J Pharm Sci. 2020; 29(1): 143-153. https://doi.org/10.31351/vol29iss1pp143-153
Badr-Eldin SM, Labib GS, Aburahma MH. Eco-friendly Tadalafil surfactant-free dry emulsion tablets (SFDETs) stabilized by in situ self-assembled aggregates of natural oil and native Cyclodextrins. American association of pharmaceutical scientists. 2019 ; 20(7): 1-12. https://doi.org/10.1208/s12249-019-1450-8
Jug M, Yoon BK, Jackman JA. Cyclodextrin-based Pickering emulsions: functional properties and drug delivery applications. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2021 ; 101(1-2): 31-50. https://doi.org/10.1007/s10847-021-01097-z
Kolawole OM, Akinlabi KQ, Silva BO. Physicochemical and stability profile of Castor oil emulsions stabilized using natural and synthetic emulsifiers. World j of biology pharmacy and health sciences. 2022 ; 9(2): 60-73 https://doi.org/10.30574/wjbphs.2022.9.2.0043
Shirajuddin S.S.M, Al bakri Abdullah M.M. , Ghazali C.M.r., et al. Study on the emulsion stability of tripropylene Glycol diacrylate in water. Archives of metallurgy and materials. 2023 ; 68(3): 1011-1016. https://doi.org/10.24425/amm.2023.145468
Dahash RA, Rajab NA. Formulation and investigation of lacidipine as nanoemulsions. Iraqi J Pharm Sci. 2020; 29(1): 41-54. https://doi.org/10.31351/vol29iss1pp41-54
Teh SS, Mah SH. Stability evaluations of different types of vegetable oil-based emulsions. J of Oleo Science. 2018; 67(11): 1381-1387. https://doi.org/10.5650/jos.ess18067
Bamiro O.A, Eke C.P, Alayo M.A. The emulsifying properties of Terminalia Randii Baker F. gum in Castor oil and liquid Paraffin emulsions. Nigerian J of Pharmaceutical Research. 2016; 12(2): 155-162. https://doi.org/10.26226/morressier.57d6b2bad462b8028d88cf40
Taher SS, Al-Kinani KK, Hammoudi ZM, et al. Co-surfactant effect of polyethylene glycol 400 on microemulsion using BCS class II model drug. J of Advanced Pharmacy Education & Research. 2022; 12(1): 63-69. https://doi.org/10.51847/1h17TZqgyI
Motka U, Dabhi M, Sheth N, et al. Formulation and optimization of nanosuspension prepared by media milling technique to enhance the solubility of Isradipine. International J of pharmaceutical sciences and drug research. 2017; 9(4):169-177. https://doi.org/10.25004/IJPSDR.2017.090403
Hamzah ZO, Ali WK. Utilization of natural polyelectrolytes in the preparation of Naproxen as sustained release matrix tablet. Al Mustansiriyah J of pharmaceutical sciences. 2019; 19(2): 17-29. https://doi.org/10.32947/ajps.19.02.0400
Suzi HM, Al-Khedairy EBH. Formulation and in vitro evaluation of taste-masked Prednisolone orodispersible tablets. J Fac Med Baghdad. 2023 ; 65(3): 192-198. https://doi.org/10.32007/jfacmedbagdad.2057
Loi CC, Eyres GT, Silcock P, et al. Preparation and characterization of a novel emulsifier system based on glycerol monooleate by spray-drying. J of Food Engineering. 2020 ; 285(8): 1-7. https://doi.org/10.1016/j.jfoodeng.2020.110100
Kmkm AM, Ghareeb MM. Natural oil nanoemulsion-based gel vehicle for enhancing antifungal effect of topical Luliconazole. J Fac Med Baghdad. 2023 ; 65(1): 65-73..
https://doi.org/10.32007/jfacmedbagdad.6512058
Sun Z, Yan X , Xiao Y , et al. Pickering emulsions stabilized by colloidal surfactants: role of solid particles. Particuology. 2022 ; 64: 153-163.https://doi.org/10.1016/j.partic.2021.06.004
Kolahi P, Shekarchizadeh H, Nasirpour A. Stabilization of Pickering emulsion using tragacanth nanoparticles produced by a combination of ultrasonic and anti‐solvent methods. J of the science of food and agriculture. 2021;102(4):1353-62. https://doi.org/10.1002/jsfa.11467
Muhammed SA, Al-Kinan KK. Formulation and in vitro evaluation of Meloxicam as a self-microemulsifying drug delivery system. F1000Research. 2023 ; 12(315): 1-24.
https://doi.org/10.12688/f1000research.130749.2
Song Z, Yang Y, Chen F, et al. Effects of concentration of Soybean protein isolate and Maltose and oil phase volume fraction on freeze-thaw stability of Pickering emulsion. Foods. 2022 ; 11(24): 1-15. https://doi.org/10.3390/foods11244018
Taarji N, Bouhoute M, Melanie H, et al. Stability characteristics of O/W emulsions prepared using purified glycyrrhizin or a non-purified glycyrrhizin-rich extract from liquorice root (Glycyrrhiza glabra). Colloids and surfaces A: Physicochemical and engineering aspects. 2021;614:126006. https://doi.org/10.1016/j.colsurfa.2020.126006
Dong B, Qin Z, Wang Y, et al. Investigating the rheology and stability of heavy crude oil-in-water emulsions using APG08 emulsifiers. ACS Omega. 2022 ; 7(42): 37736-37747.
https://doi.org/10.1021/acsomega.2c04684
Li Q, Huang Y, Du Y, et al. Food-grade olive oil pickering emulsions stabilized by starch/β-cyclodextrin complex nanoparticles: Improved storage stability and regulatory effects on gut microbiota. LWT. 2022;155:112950. https://doi.org/10.1016/j.lwt.2021.112950
Li W, Jiao B, Li S, et al. Recent advances on Pickering emulsions stabilized by diverse edible particles: stability mechanism and applications. Frontiers in nutrition. 2022 ; 9: 1-17.. https://doi.org/10.3389/fnut.2022.864943.
Esparza Y, Ngo T-D, Boluk Y. Preparation of powdered oil particles by spray drying of cellulose nanocrystals stabilized Pickering hempseed oil emulsions. Colloids and surfaces A: Physicochemical and engineering Aspects. 2020;598:124823. https://doi.org/10.1016/j.colsurfa.2020.124823
Burgos-Díaz C, Garrido-Miranda KA, Palacio DA. Food-grade oil-in-water (O/W) Pickering emulsions stabilized by Agri-food byproduct particles. Colloids and interfaces. 2023; 7(27).
https://doi.org/10.3390/colloids7020027
Taylor K, Aulton A. Aulton's pharmaceutics. 6th ed. UK: Elsevier; 2022.
British pharmacopoeia. London: Medicines and Healthcare products. Regulatory Agency, 2016.p741.
Taher MN, Hussein AA. Formulation and evaluation of Domperidone nanoemulsions for oral rout. Iraqi J Pharm Sci,. 2015; 24(2): 77-90. https://doi.org/10.31351/vol24iss2pp77-90
Shitole M, Vt P. Study of potential antitussive activity of Glycyrrhiza Glabra granules using a cough model induced by Sulfur dioxide gas in mice. Asian J of pharmaceutical and clinical research. 2019; 12(10): 262-267. https://doi.org/10.22159/ajpcr.2019.v12i10.33967
التنزيلات
منشور
إصدار
القسم
الرخصة
الحقوق الفكرية (c) 2024 Zahraa M. Al-Sharify, Fatima J. Jawad

هذا العمل مرخص بموجب Creative Commons Attribution 4.0 International License.




















 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


