Arrestin Beta-2 in Prostate Cancer and its Relationship with Trace Elements
DOI:
https://doi.org/10.32007/jfacmedbaghdad2476Keywords:
Arrestin Beta -2, Copper, Prostate cancer, Manganese, ZincAbstract
Background: Prostate cancer (PCa) is the most prevalent non-cutaneous malignancy and the second-leading cause of cancer-relatedmortality among males. Cancer is just one of the several disorders that may be linked to variations in trace element concentrations.
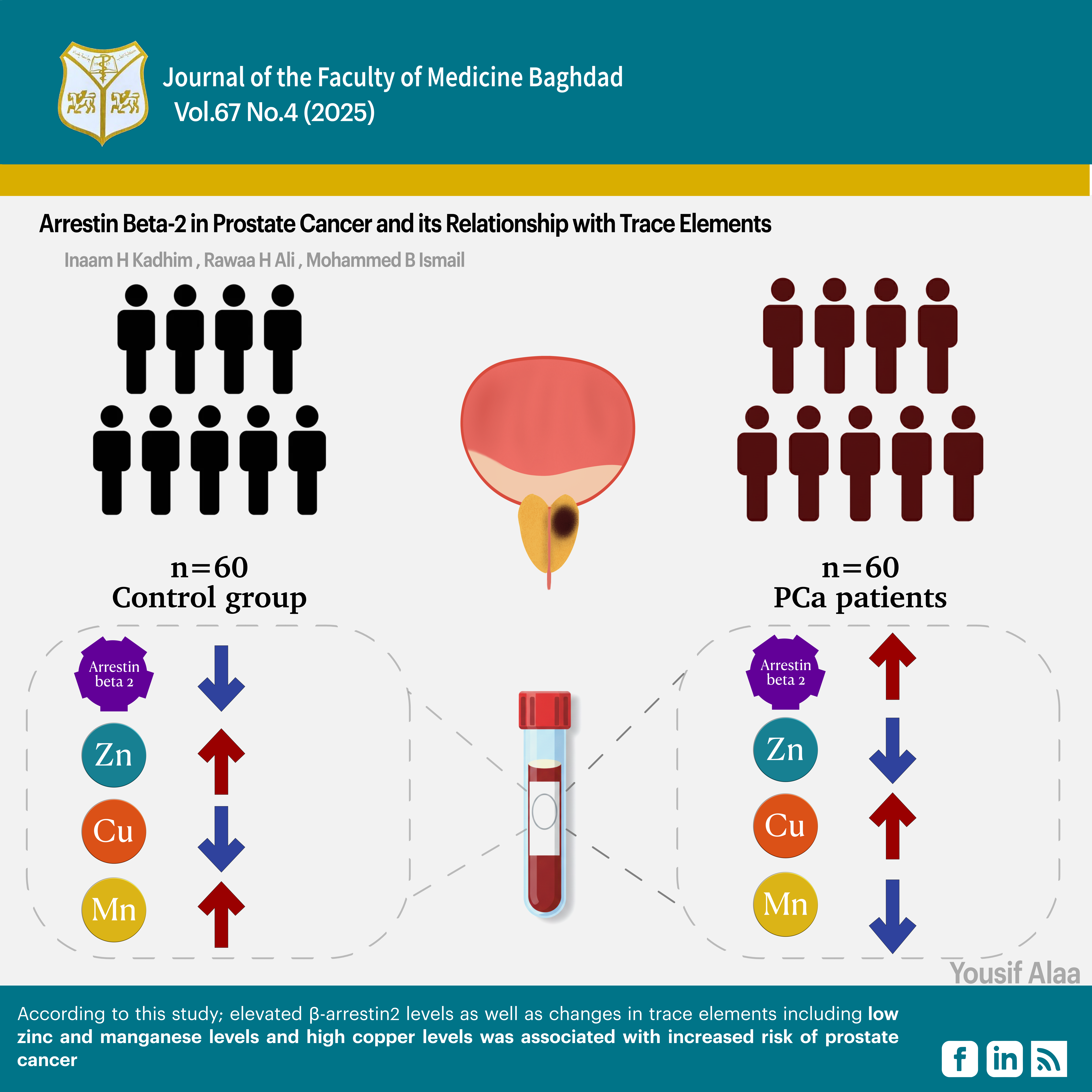
Objective: To evaluate serum arrestin β-2 as a prostate cancer tumor marker and its association with trace elements.
Methods: This case- control study was carried out at the Department of Biochemistry, College of Medicine, University of Baghdad and at the Urology department, Ghazi Al-Hariri Hospital for Surgical Specialities during the period from March 2022 to May 2023. In this case-control study 120 males were enrolled: Sixty men with newly diagnosed primary prostate cancer (PCa) and 60 healthy men as controls. Blood samples were tested for arrestin β-2 levels using an enzyme-linked immunosorbent assay (ELISA), and trace element levels, including Zinc, Copper, and Manganese, were measured using a flame atomic absorption spectrophotometer.
Results: Arrestin β-2 levels differed significantly between the control group and PCa patients. The serum copper level was significantly higher in the cases than in the controls. Zinc and manganese levels were significantly higher in the controls compared to the patients.
Conclusion: It can be concluded that high arrestin β-2 and Copper levels and low Zinc and Manganese levels may serve as potential biomarkers for patients with prostatic Adenocarcinoma.
Received: Sept. 2024
Revised: Nov. 2025
Accepted: Dec. 2025
Published Online: Dec. 2025
Published Dec. 2025
Downloads
References
1. Kadhim IH, Ali RH, Ismail MB. Ceramide As A Potential Tumor Marker For Diagnosis Of Prostate Cancer And Its Association With Lipid Profile. SEEJPH. 2024; 24special(52): 520 https://doi.org/10.70135/seejph.vi.1012. DOI: https://doi.org/10.70135/seejph.vi.1012
2. Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate Cancer Nat Rev Dis Primers. 2021; 7 (1): 9. https://doi.org/10.1038/s41572-020-00243-0. DOI: https://doi.org/10.1038/s41572-020-00243-0
3. Kallifatidis G, Mamouni K, Lokeshwar BL. The role of β-arrestins in regulating stem cell phenotypes in normal and tumorigenic cells. Int J Mol Sci. 2020;21(23):9310. https://doi.org/10.3390/ijms21239310. DOI: https://doi.org/10.3390/ijms21239310
4. allifatidis G, Smith DK, Morera DS, Gao J, Hennig MJ, Hoy JJ, et al. β-Arrestins regulate stem cell-like phenotype and response to chemotherapy in bladder cancer. Mol Cancer Ther. 2019;18(4):801-11. https://doi.org/10.1158/1535-7163.MCT-18-1167. DOI: https://doi.org/10.1158/1535-7163.MCT-18-1167
5. Kee TR, Khan SA, Neidhart MB, Masters BM, Zhao VK, Kim YK, et al. The multifaceted functions of β-arrestins and their therapeutic potential in neurodegenerative diseases. Exp Mol Med. 2024;56(1):129-41. https://doi.org/10.1038/s12276-023-01144-4. DOI: https://doi.org/10.1038/s12276-023-01144-4
6. Habrowska-Górczyńska DE, Kozieł MJ, Kowalska K, Piastowska-Ciesielska AW. FOXO3a and its regulators in prostate cancer. Int J Mol Sci. 2021;22(22):12530. https://doi.org/10.3390/ijms222212530. DOI: https://doi.org/10.3390/ijms222212530
7. Chasapis CT, Ntoupa PSA, Spiliopoulou CA, Stefanidou ME. Recent aspects of the effects of zinc on human health. Arch Toxicol. 2020; 94:1443-60. https://doi.org/10.1007/s00204-020-02702-9. DOI: https://doi.org/10.1007/s00204-020-02702-9
8. Golonka RM, Xiao X, Abokor AA, Joe B, Vijay-Kumar M. Altered nutrient status reprograms host inflammation and metabolic health via gut microbiota. J Nutr Biochem. 2020; 80:108360. https://doi.org/10.1016/j.jnutbio.2020.108360. DOI: https://doi.org/10.1016/j.jnutbio.2020.108360
9. Ahmed NI, Ali RH, Ismail MB. Kidney functions and electrolyte disturbance among Iraqi patients with bladder cancer. J Fac Med Baghdad [Internet]. 2023 Jan. https://doi.org/10.32007/jfacmedbagdad.6441985. DOI: https://doi.org/10.32007/jfacmedbagdad.6441985
10. Li D, Stovall DB, Wang W, Sui G. Advances of zinc signaling studies in prostate cancer. Int J Mol Sci. 2020;21(2):667. https://doi.org/10.3390/ijms21020667. DOI: https://doi.org/10.3390/ijms21020667
11. Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32(5):417-8. https://doi.org/10.1038/s41422-022-00653-7. DOI: https://doi.org/10.1038/s41422-022-00653-7
12. Lu Y, Su H, Wang Y, Li H. Micronutrients and risks of three main urologic cancers: A Mendelian randomization study. Front Nutr. 2023; 10:1016243. https://doi.org/10.3389/fnut.2023.1016243. DOI: https://doi.org/10.3389/fnut.2023.1016243
13. Saleh SAK, Adly HM, Abdelkhaliq AA, Nassir AM. Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr Urol. 2020;14(1):44-9. https://doi.org/10.1159/000499261. DOI: https://doi.org/10.1159/000499261
14. Baidya M, Kumari P, Dwivedi‐Agnihotri H, Pandey S, Chaturvedi M, Stepniewski TM, et al. Key phosphorylation sites in GPCRs orchestrate the contribution of β‐Arrestin 1 in ERK 1/2 activation. EMBO Rep. 2020;21(9): e49886. https://doi.org/10.15252/embr.201949886. DOI: https://doi.org/10.15252/embr.201949886
15. Hu S, Wang D, Wu J, Jin J, Wei W, Sun W. Involvement of β-arrestins in cancer progression. Mol Biol Rep. 2013; 40:1065-71. https://doi.org/10.1007/s11033-012-2148-0. DOI: https://doi.org/10.1007/s11033-012-2148-0
16. Lakshmikanthan V, Zou L, Kim JI, Michal A, Nie Z, Messias NC, et al. Identification of βArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci. 2009;106(23):9379-84. https://doi.org/10.1073/pnas.0900258106. DOI: https://doi.org/10.1073/pnas.0900258106
17. Wu S, Li M, Chen F, Zeng Y, Xu C. Inhibition of β2-adrenergic receptor regulates necroptosis in prostate cancer cell. Heliyon. 2024;10(11). https://doi.org/10.1016/j.heliyon.2024.e31865. DOI: https://doi.org/10.1016/j.heliyon.2024.e31865
18. Lin YH, Lin YC, Chen CC. Lysophosphatidic acid receptor antagonists and cancer: the current trends, clinical implications, and trials. Cells. 2021;10(7):1629. https://doi.org/10.3390/cells10071629. DOI: https://doi.org/10.3390/cells10071629
19. Kim WK, Lee Y, Jang SJ, Hyeon C. Kinetic model for the desensitization of G protein-coupled receptor. J Phys Chem Lett. 2024;15:6137-45. https://doi.org/10.1021/acs.jpclett.4c00967. DOI: https://doi.org/10.1021/acs.jpclett.4c00967
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Inaam H. Kadhim, Rawaa H. Ali, Mohammed B. Ismaeel

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


