Proenkephalin-A as a Biomarker for Type 2 Diabetes in Women with Thyroid Disorders
DOI:
https://doi.org/10.32007/jfacmedbaghdad3162Keywords:
Diabetes Mellitus; , Hyperthyroidism; , Hypothyroidism; , Proenkephalin-A; , Thyroid disorderAbstract
Background: The thyroid is a vital endocrine gland that regulates growth, metabolism, and development. Thyroid dysfunction (TD) and diabetes mellitus (DM) are two prevalent endocrine disorders with overlapping pathophysiological features.
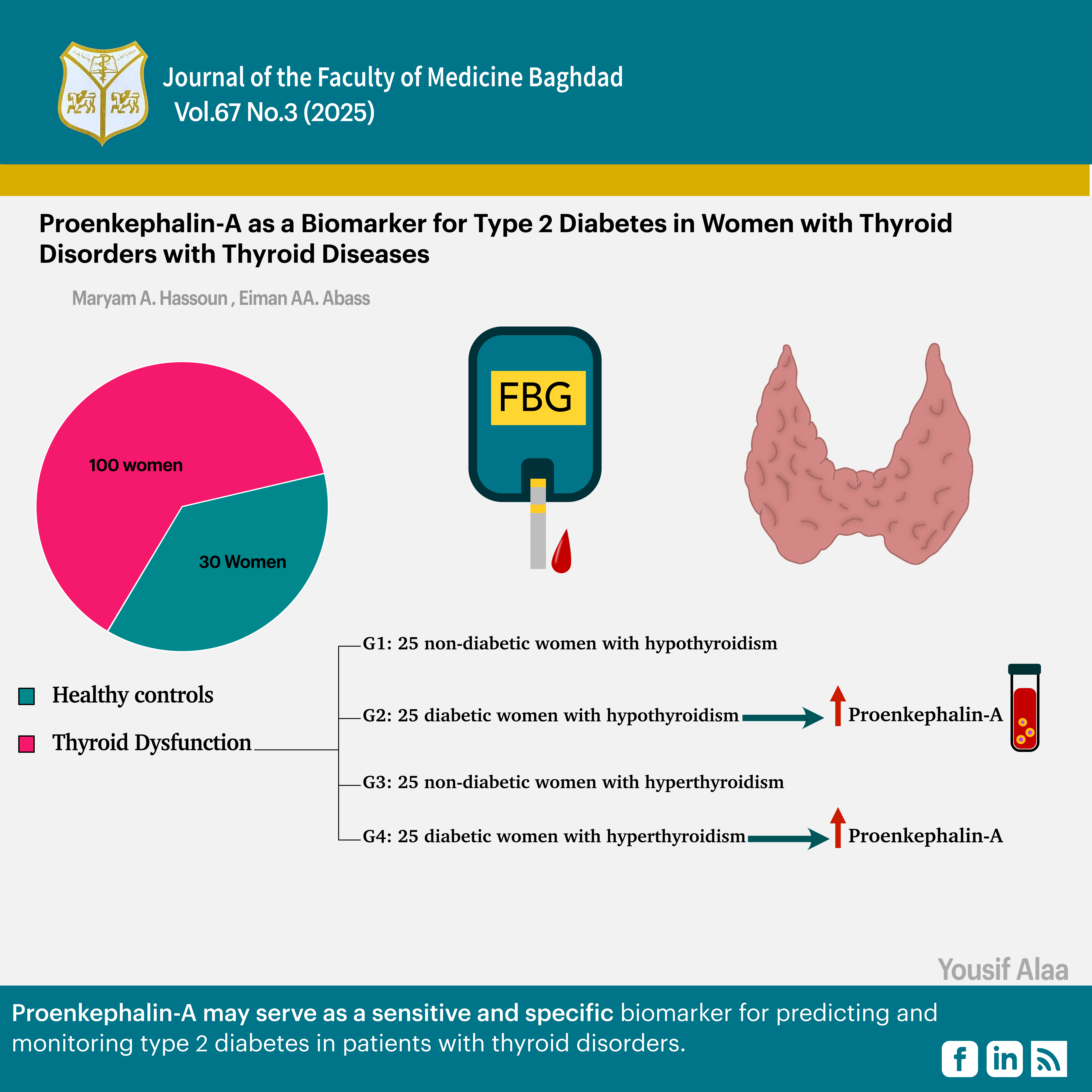
Objective: To investigate the potential role of proenkephalin-A (PENK-A) as a diagnostic and predictive biomarker for type 2 diabetes in women with thyroid dysfunction.
Methods: A total of 130 women aged 25–55 years were enrolled in the study, including 100 patients with thyroid disorders and 30 healthy controls. The study was conducted from December 2024 to April 2025 at Al-Kindy Center for Endocrinology and Diabetes in Baghdad. Participants were categorized into four subgroups based on the presence or absence of hypothyroidism, hyperthyroidism, and DM. Serum levels of PENK-A, TSH, T3, T4, and HbA1c were measured using different principles of the ELISA technique, while (FBG) Fasting Blood Glucose was determined by the enzymatic colorimetric method using a glucose kit from Randox. To express the data, the mean ±SD was used.
Results: PENK-A levels were significantly elevated in patients with both thyroid dysfunction and type 2 diabetes compared to non-diabetic patients and controls. PENK-A demonstrated high diagnostic performance in distinguishing hypothyroidism and hyperthyroidism patients with DM from those without DM, achieving an outstanding AUC of 0.942 and 0.813, respectively.
Conclusions: Proenkephalin-A may serve as a sensitive and specific biomarker for predicting and monitoring type 2 diabetes in patients with thyroid disorders.
Received: June 2025
Revised: Sept. 2025
Accepted: Sept. 2025
Published Online: Sept. 2025
Published: Sept. 2025
References
1. Das D, Banerjee A, Jena AB, Duttaroy AK, Pathak S. Essentiality, relevance, and efficacy of adjuvant/combinational therapy in the management of thyroid dysfunctions. Biomedicine & Pharmacotherapy. 2022;146:112613.;146:112613. https://doi.org/10.1016/j.biopha.2022.112613. DOI: https://doi.org/10.1016/j.biopha.2022.112613
2. Saleh DS, Othman MS. Exploring the challenges of diagnosing thyroid disease with imbalanced data and machine learning: a systematic literature review. Baghdad Sci J. 2024;21(3):1119-. DOI: https://doi.org/10.21123/bsj.2023.8544). DOI: https://doi.org/10.21123/bsj.2023.8544
3. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-421. https://doi.org/10.1089/thy.2016.0229 DOI: https://doi.org/10.1089/thy.2016.0229
4. Concepción-Zavaleta MJ, Coronado-Arroyo JC, Quiroz-Aldave JE, Durand-Vásquez MD, Ildefonso-Najarro SP, Rafael-Robles LD, et al. Endocrine factors associated with infertility in women: An updated review. Expert review of endocrinology & metabolism.2023;18(5):399-417. https://doi.org/10.1080/17446651.2023.2256405 DOI: https://doi.org/10.1080/17446651.2023.2256405
5. Al–A'araji SB, Rasen TF, Kadhum RA. Biochemical Study on Anti Thyroid Peroxidase in Type 2 Diabetic patients with thyroid disorders. Baghdad Sci J. 2016;13(4):0753-. https://doi.org/10.21123/bsj.2016.13.4.0753. DOI: https://doi.org/10.21123/bsj.2016.13.4.0753
6. Abubakar MZ, Abdulsalam K, Yahaya IA. Thyroid hormones profile of patients with type 2 diabetes mellitus in Kano, Nigeria. Annals of African Medical Research. 2020;3(1). https://doi.org/10.4081/aamr.2020.112 DOI: https://doi.org/10.4081/aamr.2020.112
7. Rong F, Dai H, Wu Y, Li J, Liu G, Chen H, et al. Association between thyroid dysfunction and type 2 diabetes: a meta-analysis of prospective observational studies. BMC medicine. 2021; 19:1-3. https://doi.org/10.1186/s12916-021-02121-2 DOI: https://doi.org/10.1186/s12916-021-02121-2
8. Abass EA. Vitamin D level and its relation with the newly diagnosed diabetic neuropathy in women with hypothyroidism. Archives of Razi Institute. 2022; 77(3):1139. https://doi.org/10.22092/ari.2022.357389.2029
9. Badr HR, Shaban MA, Gazala EM. Thyroid Diseases As a Risk of Type 2 Diabetes Mellitus. Menoufia Medical Journal. 2023;36(3):1.DOI: https://doi.org/10.59204/2314-6788.1035) DOI: https://doi.org/10.59204/2314-6788.1035
10. Vemula SL, Aramadaka S, Mannam R, Narayanan RS, Bansal A, Yanamaladoddi VR, et al. The impact of hypothyroidism on diabetes mellitus and its complications: a comprehensive review. Cureus. 2023 ;15(6). https://doi.org/10.7759/cureus.40447 DOI: https://doi.org/10.7759/cureus.40447
11. Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Therapy. 2019;10(6):2035-44. https://doi.org/10.6084/m9.figshare.9878615 DOI: https://doi.org/10.1007/s13300-019-00700-4
12. Khan SH, Fazal N, Ijaz A, Manzoor SM, Asif N, Rafi T, et al. Insulin resistance and glucose levels in subjects with subclinical hypothyroidism. J Coll PhysiciansSurgPak.2017;27(6):32933.DOI:https://doi.org/pubmed.ncbi.nlm.nih.gov/28689519
13. Sutkowska E, Kisiel M, Zubkiewicz-Kucharska A. When Should the Treatment of Obesity in Thyroid Disease Begin?. Biomedicines. 2025;13(1):157. https://doi.org/10.3390/biomedicines13010157 DOI: https://doi.org/10.3390/biomedicines13010157
14. Rahmah AM, Al-Isawi JK, Mahdi OA. The efficacy of once-daily liraglutide as an add-on to oral antidiabetic agents on weight reduction and glycemic control in obese patients with inadequately controlled type 2 diabetes: a retrospective analysis in relation to liraglutide dose escalation within a 7-month treatment period. International Journal of Diabetes in Developing Countries. 2021;41(2):266-72. DOI: https://link.springer.com/article/10.1007/s13410-020-00878-5 DOI: https://doi.org/10.1007/s13410-020-00878-5
15. Beunders R, Struck J, Wu AH, Zarbock A, Di Somma S, Mehta RL, et al. Proenkephalin (PENK) as a novel biomarker for kidney function. The journal of applied laboratory medicine. 2017 ;2(3):400- 12. DOI: https://doi.org/10.1373/jalm.2017.023598. DOI: https://doi.org/10.1373/jalm.2017.023598
16. Marino R, Struck J, Hartmann O, Maisel AS, Rehfeldt M, Magrini L, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. Journal of nephrology. 2015 ;28(6):717-24. https://doi.org/10.1007/s40620-014-0163-z DOI: https://doi.org/10.1007/s40620-014-0163-z
17. Breidthardt T, Jaeger C, Christ A, Klima T, Mosimann T, Twerenbold R, et al. Proenkephalin for the early detection of acute kidney injury in hospitalized patients with chronic kidney disease. European journal of clinical investigation. 2018;48(10):e12999. https://doi.org/10.1111/eci.12999 DOI: https://doi.org/10.1111/eci.12999
18. Denning GM, Ackermann LW, Barna TJ, Armstrong JG, Stoll LL, Weintraub NL, et al. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008 ;29(1):83-92. https://doi.org/10.1016/j.peptides.2007.11.004 DOI: https://doi.org/10.1016/j.peptides.2007.11.004
19. Schulz CA, Christensson A, Ericson U, Almgren P, Hindy G, Nilsson PM, et al. High level of fasting plasma proenkephalin-A predicts deterioration of kidney function and incidence of CKD. Journal of the American Society of Nephrology. 2017 ;28(1):291-303. https://doi.org/10.1681/ASN.2015101177 DOI: https://doi.org/10.1681/ASN.2015101177
20. Massod MF. Nonparametric percentile estimate of clinical normal ranges. The American journal of medical technology. 1977 ;43(3):243-52.
21. Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes care. 1995;18(6):896-909. https://doi.org/10.2337/diacare.18.6.896 DOI: https://doi.org/10.2337/diacare.18.6.896
22. Elghoneimy YF, Nashy MR, Othman SA, Almarri NM, Alruwaili AA, Alotaibi AR, et al. Which level of preoperative glycated haemoglobin (HbA1c) affect early morbidity and mortality after cardiac surgery?. Australasian Medical Journal (Online). 2020;13(1):32-40. https://doi.org/10.35841/1836-1935.13.1.32-40 DOI: https://doi.org/10.35841/1836-1935.13.1.32-40
23. Kumar R, Saha P, Kumar Y, Sahana S, Dubey A, Prakash O. A review on diabetes mellitus: type1 & Type2. World Journal of Pharmacy and Pharmaceutical Sciences. 2020;9(10):838-50. DOI: https://doi.org/10.20959/wjpps202010-17336
24. Eom YS, Wilson JR, Bernet VJ. Links between thyroid disorders and glucose homeostasis. Diabetes & Metabolism Journal. 2022 ;46(2):239-56.DOI: https://doi.org/10.4093/dmj.2022.0013 DOI: https://doi.org/10.4093/dmj.2022.0013
25. Lambadiari V, Mitrou P, Maratou E, Raptis AE, Tountas N, Raptis SA, et al. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine. 2011;39(1):28-32.
https://doi.org/10.1007/s12020-010-9408-3. DOI: https://doi.org/10.1007/s12020-010-9408-3
26. Yang W, Jin C, Wang H, Lai Y, Li J, Shan Z. Subclinical hypothyroidism increases insulin resistance in normoglycemic people. Frontiers in Endocrinology. 2023;14:1106968. https://doi.org/10.3389/fendo.2023.1106968 DOI: https://doi.org/10.3389/fendo.2023.1106968
27. Iedan MA, Al-Rubaei ZM. Chitotriosidase-1 levels in Iraqi Type 2 Diabetic Patients with Thyroid Disorders. Ibn AL-Haitham Journal for Pure and Applied Sciences. 2019;32(2):45-50. https://doi.org/10.30526/32.2.2138 DOI: https://doi.org/10.30526/32.2.2138
28. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. The Journal of Clinical Endocrinology & Metabolism. 2002;87(3):1068-72. https://doi.org/10.1210/jcem.87.3.8165 DOI: https://doi.org/10.1210/jc.87.3.1068
29. Al-Shaibani AB, Al-A’araji SB, Al-Mofarji ST. Studying association between thyroid disorders and Helicobacter pylori infection in Iraqi Patients. Baghdad Sci J. 2014;11(4):1528-41.DOI: https://doi.org/10.21123/bsj.2014.11.4.1528-1541. DOI: https://doi.org/10.21123/bsj.2014.11.4.1528-1541
30. Krotkiewski M. Thyroid hormones in the pathogenesis and treatment of obesity. European journal of pharmacology. 2002;440(2-3):85-98. https://doi.org/10.1016/S0014-2999(02)01420-6 DOI: https://doi.org/10.1016/S0014-2999(02)01420-6
31. Jabar SS, Mohammed SB, Mahdi AR. Level and Statistical Distribution of Thyroid Peroxidase and Thyroid Hormones in Iraqi patients with Type1 Diabetes Mellitus at Al-Karkh Side. Baghdad Sci J. 2016;13(2):0312-. https://doi.org/10.21123/bsj.2016.13.2.0312 DOI: https://doi.org/10.21123/bsj.2016.13.2.0312
32. Dawood AS, Abed BA, Farhan LO, Salman IN. Evaluation of Neudesin Level in A sample of Iraqi Patients with Hypothyroidism. Iraqi Journal of Science. 2024:6205-13.DOI: https://doi.org/10.24996/ijs.2024.65.11.1 DOI: https://doi.org/10.24996/ijs.2024.65.11.1
33. Abass EA, Warka’a T, Moslem MN. A comparative study of retinol-binding protein-4 and progranulin in iraqi women with thyroid disorder. parameters. 2021;1(25):G2. https://doi.org/10.25258/ijddt.11.1.6
34. Al-Rubaei ZM, Abdulhadi GH, Alrubaye IM, Alrugaibi AH. Comparative study of fetuin-aleves in Iraqi diabetic patients with hyper and thyroid disorder. Biochemical & Cellular Archives. 2020 ;20(2). DOI: https://connectjournals.com/03896.2020.20.5027
35. Abed BA, Salman IN, Hassan EA, Mohammed NU. Role of stanniocalcin-1 and proenkephalin-A as novel biomarkers in prediction of newly diagnosed type 2 diabetic patients. International Journal of Diabetes in Developing Countries. 2024;20:1-7. https://doi.org/10.1007/s13410-024-01353-1 DOI: https://doi.org/10.1007/s13410-024-01353-1
36. Green IC, Perrin D, Pedley KC, Leslie RD, Pyke DA. Effect of enkephalins and morphine on insulin secretion from isolated rat islets. Diabetologia. 1980;19:158-61. https://doi.org/10.1007/BF00421864 DOI: https://doi.org/10.1007/BF00421864
37. Schleicher RL, Chawla RK, Coan PA, Martino-Saltzman D, Collins DC. Beta-endorphin-induced hyperglycemia in rabbits: effects of a glucose or arginine challenge. American Journal of Physiology-Endocrinology and Metabolism. 1987;252(2):E255-9. https://doi.org/10.1152/ajpendo.1987.252.2.E255 DOI: https://doi.org/10.1152/ajpendo.1987.252.2.E255
38. Gupta A, Gullapalli S, Pan H, Ramos-Ortolaza DL, Hayward MD, Low MJ, et al. Regulation of opioid receptors by their endogenous opioid peptides. Cellular and Molecular Neurobiology. 2021;41(5):1103-18.
https://doi.org/10.1007/s10571-020-01015-w DOI: https://doi.org/10.1007/s10571-020-01015-w
39. Escolero V, Tolentino L, Muhammad AB, Hamid A, Lutfy K. The Involvement of Endogenous Enkephalins in Glucose Homeostasis. Biomedicines. 2023;11(3):671. https://doi.org/10.3390/biomedicines11030671 DOI: https://doi.org/10.3390/biomedicines11030671
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Maryam A. Hassoun, Eiman A.A. Abass

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


