The Anti-Inflammatory Effect of Chenopodium Murale in Comparison to Salvia Frigida on Atopic Eczema
DOI:
https://doi.org/10.32007/jfacmedbaghdad.6642371Keywords:
Atopic dermatitis, Chenopodium murale, Phenolic compound, Salvia frigida, TacrolimusAbstract
Background: Atopic dermatitis (AD) is a prevalent chronic inflammatory skin condition with a familial tendency. It affects approximately 10%-20% of children and 1%-3% of adults worldwide. Chenopodium murale is clinically proven for treating many medical conditions, such as AD, due to its easy application and efficacy. Salvia plant has an anti-inflammatory effect on AD cases treated with phenolic compounds.
Objective: To determine the anti-inflammatory effect of the phytosterol fraction of Chenopodium murale (CM) in comparison to Salvia frigida (SF).
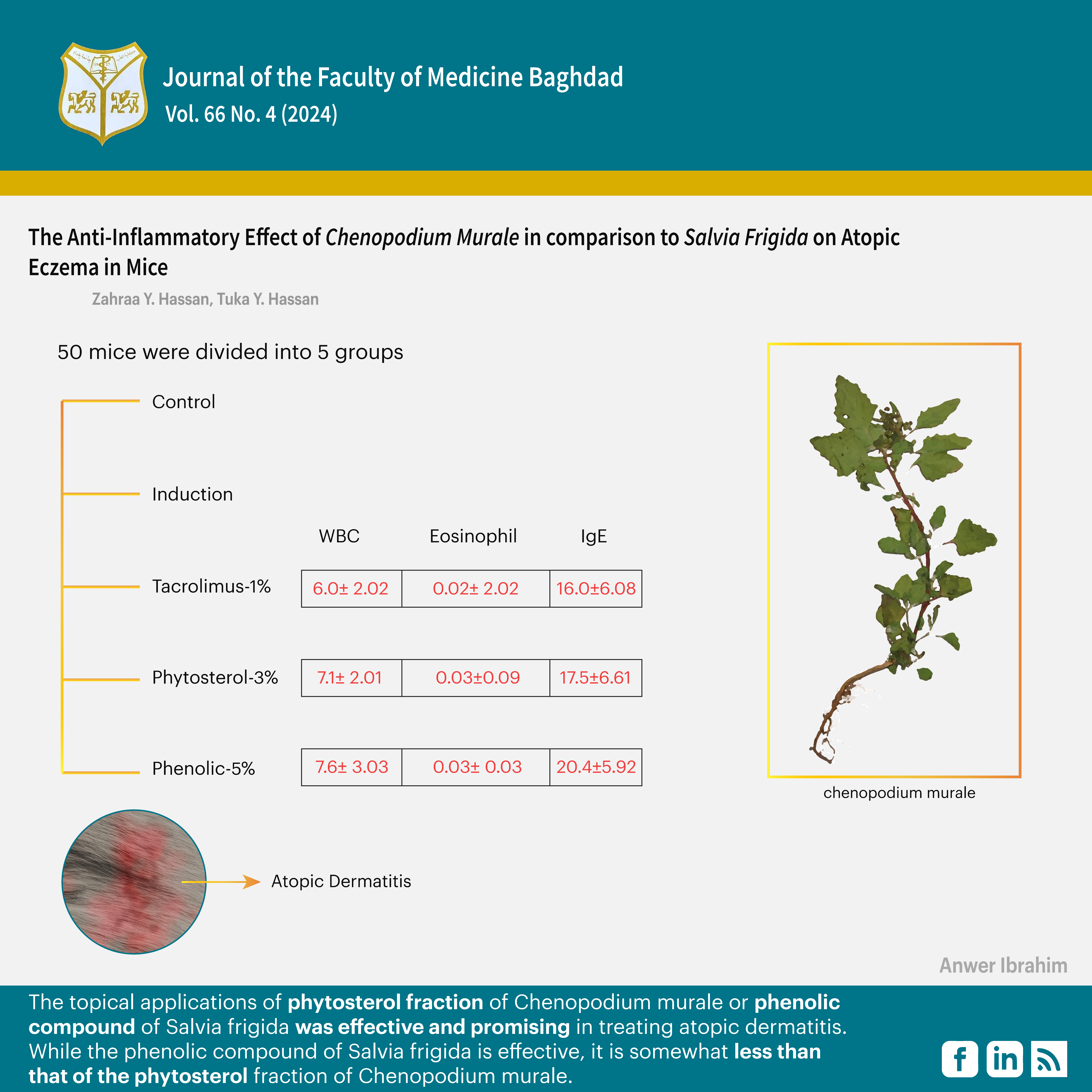
Methods: This study was conducted from December 2020 to June 2021 in the Department of Pharmacology, College of Medicine, Al Nahrain University. Fifty mice were included in the study, subdivided equally into five subgroups [control, induction, Tacrolimus-1%, Phytosterol-3%, and Phenolic-5%]. Biological and histological parameters were measured, and their means were compared using the independent t-test, and the one-way ANOVA was used to estimate the mean of differences.
Results: The Tacrolimus-1% group showed a significant decrease in white blood cells, Ig-E, and inflammation means than other groups; a significant decrease in mean epidermal thickness than the Phytosterol-3% groups; and a significant decrease in IL-13 and erosion than the Phenolic-5% groups. The phytosterol-3% group showed a significant decrease in the mean parakeratosis, erosion, and observational severity (OS) score than other groups. The phenolic-5% group showed a significant decrease in the mean epidermal thickness than other groups and a significant decrease in OS score than the Tacrolimus-1% groups.
Conclusion: The topical applications of the phytosterol fraction of Chenopodium murale or the phenolic compound of Salvia frigida were effective and promising in treating atopic dermatitis. While the phenolic compound of Salvia frigida is effective, it is somewhat less than that of the phytosterol fraction of Chenopodium murale.
Received: April 2024
Revised: Sept. 2024
Accepted: Oct. 2024
Published: Dec. 2024
Downloads
References
Ahn K, Kim BE, Kim J, Leung DY. Recent advances in atopic dermatitis. Current Opinion in Immunology, 2020;66:14-21. https://doi.org/10.1016/j.coi.2020.02.007.
Kim J, Kim BE, Leung DY. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019;40(2):84-92. https://doi.org/10.2500/aap.2019.40.4202
Tanei R. Atopic Dermatitis in Older Adults: A Review of Treatment Options. Drugs Aging 2020:37(3):149-160. https://doi.org/10.1007/s40266-020-00750-5
Mohamed AA, El Borolossy R, Salah EM, Hussein MS, Muharram NM, Elsalawy N, et al. A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front. Pharmacol. 2023; 14:1202325. https://doi.org/10.3389/fphar.2023.1202325
Ahmed AA, Abu-Raghif AR. Effect of Topical Phytosterol Fraction of Chenopodium murale on Induced Hypertrophic Scar in Rabbits. Journal of Global Pharma Technology, 2020;12(02):115-124. https://www.jgpt.co.in/index.php/jgpt/article/view/3231/2567
Abd Elkarim AS, Ahmed AH, Taie HAA, Elgamal AM, Abu-elghait M, Shabana S. Synadenium grantii hook f.: HPLC/QTOF-MS/MS tentative identification of the phytoconstituents, antioxidant, antimicrobial and antibiofilm evaluation of the aerial parts. Rasayan. J. Chem. 2021;14:811–828. https://doi.org/10.31788/RJC.2021.1426165
Hassan ZY, Hassan TY, Abu Raghif AR. Evaluation the Effect of Phytosterol Fraction of Chenopodium murale in Comparison with Tacrolimus on Mice Induced Atopic Dermatitis. Iraqi J Pharm Sci, Vol.32(1) 2023. https://doi.org/10.31351/vol32iss1pp84-91
Batcha O, Gnatoulma K, Gérard T, Laura L, Efui G, Manuel R, et al. Anti-inflammatory, antibacterial and antioxidant activities of Chenopodium ambrosioides L. (Chenopodiaceae) extracts 2021;162: 16764 - 16794. https://doi.org/10.35759/JABs.162.7
El-Newary SA, Abd Elkarim AS, Abdelwahed NAM, Omer EA, Elgamal AM, ELsayed WM. Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile. Molecules. 2023 May 24;28(11):4304. https://doi.org/10.3390/molecules28114304
Ahmed Z, Uddin Z, Shah S, Zahoor M, Alotaibi A, Shoaib M, et al. Antioxidant, antidiabetic, and anticholinesterase potential of Chenopodium murale L. extracts using in vitro and in vivo approaches. Open Chemistry. 2022;20(1): 1171-1186. https://doi.org/10.1515/chem-2022-0232
Akgül H, Korkmaz N, Dayangaç A, Sevindik M. Antioxidant Potential of Endemic Salvia absconditiflora. Turkish Journal of Agriculture - Food Science and Technology, 2020;8(10): 2222-2224. https://doi.org/10.24925/turjaf.v8i10.2222-2224.3697
Asgarpanah J. A review on the essential oil chemical profile of Salvia genus from Iran. Nat. Volatiles and Essent. Oils, 2021; 8(3): 1-28 https://doi.org/10.37929/nveo.852794
Esmaeili G, Fatemi H, Baghani avval M, Azizi M, Arouiee H, Vaezi J, et al. Diversity of Chemical Composition and Morphological Traits of Eight Iranian Wild Salvia Species during the First Step of Domestication. Agronomy. 2022; 12(10):2455. https://doi.org/10.3390/agronomy12102455
Sunar S, Korkmaz M, Sığmaz B, Ağar G. Determination of the Genetic Relationships Among Salvia Species by RAPD and ISSR Analyses. Turk J Pharm Sci. 2020;17(5):480-485. https://doi.org/10.4274/tjps.galenos.2018.24572
Al-Hussaini A, Al-Mousawi AH, Al-Musawi AHE. The ecology and geographical distribution for the species of the genus Salvia L. of labiatae in Iraq. Baghdad Sci. J., 2013; 10 (4), 1082-1087. https://doi.org/10.21123/bsj.2013.10.4.1082-1087
Hassan ZY, Hassan TY, Abu Raghif AR. Evaluation the Effectiveness of Phenolic Compound of Salvia frigida on Induced Atopic Dermatitis in Experimental Mice. Iraqi J Pharm Sci, Vol.31(1) 2022. https://doi.org/10.31351/vol31iss1pp154-166
TrivellatoGrassi L, Malheiros A, Meyre-Silva C, Buss Z, Monguilhott ED, Fröde TS, et al. From popular use to pharmacological validation: A study of the anti-inflammatory, anti-nociceptive and healing effects of Chenopodium ambrosioides extract, Journal of Ethnopharmacology 2013;145(1):127-138. https://doi.org/10.1016/j.jep.2012.10.040
Harborne, J.B. Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 5th Edition, Chapman and Hall Ltd, London, 1998;21-72. https://link.springer.com/book/10.1007/978-94-009-5570-7
Mohammed NJ, Wisam A. Ameen W A. The effect of topical finasteride in treatment of idiopathic hirsutism. AJBM 2015; 3(9):552 – 566. https://doi.org/10.18081/2333-5106/015-09/552-566
Han JS, Won KH, Chang SE, Kim JE. Tacrolimus 0.1% ointment in the treatment of allergic contact dermatitis: a new approach. Int J Dermatol, 2014;53: e470-e471. https://doi.org/10.1111/ijd.12641
Kim H, Kim JR, Kang H, Choi J, Yang H, Lee P, et al. 7,8,49-Trihydroxyisoflavone Attenuates DNCB-Induced Atopic Dermatitis-Like Symptoms in NC/Nga Mice. PLoS ONE 2014;9(8):e104938. https://doi.org/10.1371/journal.pone.0104938
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018; 5(3):93. https://doi.org/10.3390/medicines5030093
Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. J. Med. Plants Res., 2011; 5 (31P): 6697-6703. https://doi.org/10.5897/JMPR11.1404
Roberts MD, Young KC, Fox CD, Vann CG, Roberson PA, Osburn SC, et al. An optimized procedure for isolation of rodent and human skeletal muscle sarcoplasmic and myofibrillar proteins. J Biol Methods. 2020; 7(1): e127. https://doi.org/10.14440/jbm.2020.307
Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol. 2020 Jan;124(1):36-43. https://doi.org/10.1016/j.anai.2019.10.008.
Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59(4):e75-e91. https://doi.org/10.1111/ijd.14763
Liu Y, Zienkiewicz J, Qiao H, Gibson-Corley KN, Boyd KL, Veach RA, et al. Genomic control of inflammation in experimental atopic dermatitis. Sci Rep 2022;12,18891. https://doi.org/10.1038/s41598-022-23042-x.
Celakovská J, Bukac J, Ettler K, Vaneckova J, Krcmova I, Ettlerova K, et al. Evaluation of Peripheral Blood Eosinophilia in Adolescent and Adult Patients Suffering from Atopic Dermatitis and the Relation to the Occurrence of Allergy to Aeroallergens. Indian J Dermatol. 2019; 64(1):34-40. https://doi.org/10.4103/ijd.IJD_509_17.
Simon D, Von Gunten S, Borelli S, Braathen LR, Simon HU. The interleukin-13 production by peripheral blood T cells from atopic dermatitis patients does not require CD2 costimulation. Int Arch Allergy Immunol. 2003; 132(2):148-55. https://doi.org/10.1159/000073716
Ju Ho P, Jun Sung J, Ki Cheon K, Jin Tae H. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine. 2018; 43:110-119. https://doi.org/10.1016/j.phymed.2018.04.013.
Gil TY, Kang YM, Eom YJ, Hong CH, An HJ. Anti-Atopic Dermatitis Effect of Seaweed Fulvescens Extract via Inhibiting the STAT1 Pathway. Mediators Inflamm. 2019; 20193760934. https://doi.org/10.1155/2019/3760934.
He D, Wang S, Fang G, Zhu Q, Wu J, Jianling Li, et al. LXRs/ABCA1 activation contribute to the anti-inflammatory role of phytosterols on LPS-induced acute lung injury. Journal of Functional Foods. 2022; 89:104966. https://doi.org/10.1016/j.jff.2022.104966
Cardona ID, Cho SH, Leung DY. Role of bacterial superantigens in atopic dermatitis: implications for future therapeutic strategies. Am J Clin Dermatol. 2006;7(5):273-9. https://doi.org/10.2165/00128071-200607050-00001
Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the 'hygiene hypothesis': too clean to be true? Br J Dermatol. 2005;152(2):202-16. https://doi.prg/10.1111/j.1365-2133.2004.06436.x.
Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick's Dermatology in General Medicine. 7th Edition. Vol. 1, United States: The McGraw‐Hill Companies. 2008. https://doi.org/10.1111/j.1524-4725.2008.34211.x
Awad AB, Chinnam M, Fink CS, Bradford PG. beta-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine. 2007 Nov;14(11):747-54. https://doi.org/10.1016/j.phymed.2007.01.003
Askari SF, Avan R, Tayarani-Najaran Z, Sahebkar A, Eghbali S. Iranian Salvia species: A phytochemical and pharmacological update. Phytochemistry. 2021 Mar;183:112619. doi: 10.1016/j.phytochem.2020.112619.
Gad HA, Mamadalieva RZ, Khalil N, Zengin G, Najar B, Khojimatov OK, et al. GC-MS Chemical Profiling, Biological Investigation of Three Salvia Species Growing in Uzbekistan. Molecules. 2022; 27(17):5365. https://doi.org/10.3390/molecules27175365
Al-Dabbagh MA, Shihab SA, Kadhim EJ. Effects Of Phenolic Compounds Extracted from Salvia frigida On Induced Hyperuricemia In Mice. Asian J Pharm Clin Res, 2019;12(4):211-217. http://dx.doi.org/10.22159/ajpcr.2019.v12i4.32096
Alwan NK, Shakir SA, Waheeb HH. Epidemiology of Skin Diseases among Displaced People in Diyala Province. JFacMedBagdad. 2018; 60(1):52-6. https://doi.org/10.32007/jfacmedbagdad.60145
Mustafa Thamer S, Q. Yahya M. The Effect of Lenalidomide Ointment on TNF-α Tissue Levels in Mice with Imiquimod-Induced Psoriasis. JFacMedBagdad. 2023;64(4):252-60. https://doi.org/10.32007/jfacmedbagdad.6441959
Kamatou GP, van Zyl R, Van vuuren S, Viljoen A, Figueiredo A, Barroso J, et al. Chemical Composition, Leaf Trichome Types and Biological Activities of the Essential Oils of Four Related Salvia Species Indigenous to Southern Africa. Journal of Essential Oil Research. 2006;18. 72-79. https://doi.org/10.1080/10412905.2006.12067125.
Kamatou GP, Viljoen A, Steenkamp PA. (2010). Antioxidant, anti-inflammatory activities and HPLC analysis of South African Salvia species. Planta Medica. 2010;76. https://doi.org/10.1055/s-0030-1264458
Raal A, Orav A, Arak E. Composition of the essential oil of Salvia officinalis L. from various European countries. Natural product research. 2007;21(5):406-11. https://doi.org/10.1080/14786410500528478
Altun M, Ünal M, Kocagöz T, Gören AC. Essential Oil Compositions and Antimicrobial Activity of Salvia Species. Journal of essential oil-bearing plants, 2007;10 (3): 251 -258. https://doi.org/10.1080/0972060X.2007.10643550
Kürşat M, Erecevit P, Sarı A, Emre İ, Kırbağ S, Civelek Ş. The Antimicrobial Activities of Seed Fatty Acid Extracts from Some Salvia L. Species. Turkish Journal of Science and Technology, 2012;7(1):31-36. https://www.researchgate.net/publication/290606992
Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313-52. https://doi.org/10.3390/molecules15107313
Oettgen HC. Fifty years later: Emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J Allergy Clin Immunol. 2016 Jun;137(6):1631-1645. https://doi.org/10.1016/j.jaci.2016.04.009
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Zahraa Y. Hassan, Tuka Y. Hassan, Ahmed Y. Kanany

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


