Evaluation of Human β-defensin-3 Diagnostic Role in a Group of Iraqi Patients with Osteoporosis
DOI:
https://doi.org/10.32007/jfacmedbaghdad.6642386Keywords:
Calcium, Human β-defensin-3, Osteoporosis, T-score, Vitamin D3Abstract
Background: Osteoporosis (OP) is a prevalent age-related condition that increases the risk of fracture and bone fragility, as result loss of bone mass as well as micro-architectural degradation of the bone, thereby reducing the mass and strength of bone. Human β-defensin (HBD-3) is ananti-inflammatory peptide andcrucial part of the human innate immune system. Giving early therapeutic intervention for OP requires an early diagnosis.
Objectives: To evaluate the serum HBD-3 accuracy of diagnosis in patients with osteoporosis.
Methods: The study was conducted in the National Joint Center at Yarmouk Teaching Hospital in Baghdad during September - October 2023.
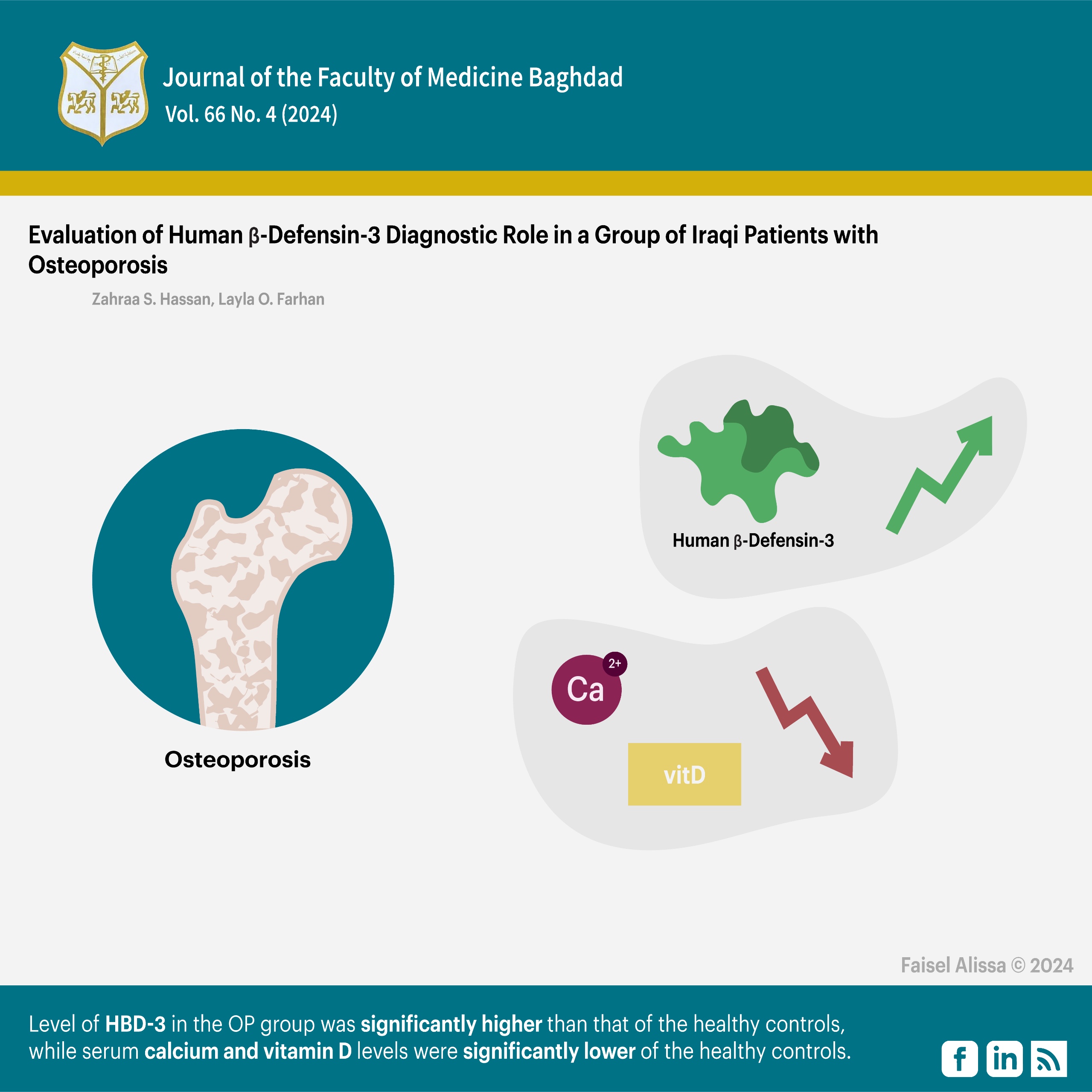
Eighty participants were recruited, all of whom had clinical examinations and had their bone status measured by dual-energy X-ray absorptiometry (DXA). Levels of serum HBD-3 and vitamin D3 (Vit D3) were determined by the ELISA technique. Calcium level (Ca+2) was measured using a spectrophotometer. A comparative study was conducted between forty patients with OP and 40 controls. The study included females and males with an age range between (40-60) years.
Results: The serum level of HBD-3 in the OP group was significantly higher (p < 0.001) than that of the healthy controls. The area under the curve (AUC) was found to be (1.000) in the ROC curve analysis for serum HBD-3 level.
Conclusion: Serum HBD-3 can be a valuable indicator of OP in middle-aged individuals, and may be a helpful biological marker for OP diagnosis.
Received: Aug. 2024
Revised: Oct. 2024
Accepted: Nov. 2024
Published: Dec. 2024
Downloads
References
1. Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105–17. https://doi.org/10.1093/bmb/ldaa005.
2. Khinda R, Valecha S, Kumar N, Walia JPS, Singh K, Sethi S, et al. Prevalence and predictors of osteoporosis and osteopenia in postmenopausal women of Punjab, India. Int J Environ Res Public Health. 2022;19(5): 1– 21. https://doi.org/10.3390/ijerph19052999.
3. Anam AK, Insogna K. Update on osteoporosis screening and management. Med Clin. 2021;105(6):1117–34. https://doi.org/10.1016/j.mcna.2021.05.016.
4. Rasmussen NH, Vestergaard P. Diabetes and osteoporosis–Treating two entities: A challenge or cause for concern? Best Pract Res Clin Rheumatol. 2022;36(3):101779. https://doi.org/10.1016/j.berh.2022.101779.
5. Llorente I, García-Castañeda N, Valero C, González-Álvaro I, Castañeda S. Osteoporosis in rheumatoid arthritis: dangerous liaisons. Front Med. 2020;7: 1–11. https://doi.org/10.3389/fmed.2020.601618.
6. Lefta NA, Abed AY, Abed BA. Estimation of asprosin levels in female Iraqi patients with type 2 diabetes and hypothyroidism. J Med Chem Sci. 2023;6(2):433–9. https://doi.org/10.26655/JMCHEMSCI.2023.2.23.
7. Zhao H, Li Y, Zhang M, Qi L, Tang Y. Blood lipid levels in patients with osteopenia and osteoporosis:a systematic review and meta-analysis. J Bone Miner Metab. 2021;39:510–20. https://doi.org/10.1007/s00774-020-01189-9.
8. Mohammed NUG, Khaleel FM, Gorial FI. The Role of Serum Chitinase-3-Like 1 Protein (YKL-40) Level and its Correlation with Proinflammatory Cytokine in Patients with Rheumatoid Arthritis. Baghdad Sci J. 2022; 19(5): 1014-1020. http://dx.doi.org/10.21123/bsj.2022.6293.
9. Farhan LO, Farhan AM, Obaidi S Al, Taha EM. A Case-control Study to Determine Metalloproteinase-12 and Lysyl Oxidase Levels in Iraqi women with Osteoporosis. Res J Pharm Technol. 2022;15(6):2655–60. https://doi.org/10.52711/0974-360X.2022.00444.
10. Zawada A, Ratajczak AE, Rychter AM, Szymczak-Tomczak A, Dobrowolska A, Krela-Kaźmierczak I. Treatment of Diabetes and Osteoporosis—A Reciprocal Risk? Biomedicines. 2022;10(9): 1–13. https://doi.org/10.3390/biomedicines10092191.
11. Hu S, Wang S, Zhang W, Su L, Ye J, Zhang D, et al. Associations between serum total cholesterol level and bone mineral density in older adults. Aging (Albany NY). 2023;15(5):1330–1342. https://doi.org/10.18632/aging.204514.
12. Chevalley T, Brandi ML, Cashman KD, Cavalier E, Harvey NC, Maggi S, et al. Role of vitamin D supplementation in the management of musculoskeletal diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group. Aging Clin Exp Res. 2022;34(11):2603–23. https://doi.org/10.1007/s40520-022-02279-6.
13. Polzonetti V, Pucciarelli S, Vincenzetti S, Polidori P. 15. Polzonetti V, Pucciarelli S, Vincenzetti S, Polidori P. Dietary Intake of Vitamin D from Dairy Products Reduces the Risk of Osteoporosis. Nutrients. 2020;12(6):1743. https://doi.org/10.3390/nu12061743.
14. Aziz ZSA, Numan S, Al-khalisy MH. Evaluation of the effect of type II diabetes mellitus on bone mineral density of upper and lower limbs by dual-energy X-ray absorptiometry. J Fac Med Baghdad. 2023;65(1):27–33. https://doi.org/10.32007/jfacmedbagdad.6511980.
15. Bertoldo F, Cianferotti L, Di Monaco M, Falchetti A, Fassio A, Gatti D, et al. Definition, assessment, and management of vitamin D inadequacy: suggestions, recommendations, and warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients. 2022;14(19): 1– 24 https://doi.org/10.3390/nu14194148.
16. De Martinis M, Allegra A, Sirufo MM, Tonacci A, Pioggia G, Raggiunti M, et al. Vitamin D deficiency, osteoporosis and effect on autoimmune diseases and hematopoiesis: a review. Int J Mol Sci. 2021;22(16): 1– 24. https://doi.org/10.3390/ijms22168855.
17. Wang L, Ran L, Zha X, Zhao K, Yang Y, Shuang Q, et al. Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. Arch Osteoporos. 2020;15:1–11. https://doi.org/10.1007/s11657-020-00833-1.
18. Abed BA, Hamid GS. Evaluation of Lipocalin-2 and Vaspin Levels in In Iraqi Women with Type 2 Diabetes Mellitus. Iraqi J Sci. 2022;4650–8 https://dx.doi.org/10.21123/bsj.2023.8119.
19. Ebeling PR, Nguyen HH, Aleksova J, Vincent AJ, Wong P, Milat F. Secondary osteoporosis. Endocr Rev. 2022;43(2):240–313. https://doi.org/10.1210/endrev/bnab028.
20. Ramazi S, Mohammadi N, Allahverdi A, Khalili E, Abdolmaleki P. A review on antimicrobial peptides databases and the computational tools. Database. 2022;2022:baac011. 1–17. https://doi.org/10.1093/database/baac011.
21. Santos CEM, Hurtado CNL, Santiago BR, Gonzalez-Amaro R, Ca˜ nizales YGC no, Martinez Fierro M de la L, et al. LL-37, HNP-1, and HBD2/3 modulate the secretion of cytokines TNF-α, IL-6, IFN-γ, IL-10 and MMP1 in human primary cell cultures. Eur Cytokine Netw. 2016;27:68–74. https://doi.org/10.1684/ecn.2016.0379.
22. Gao X, Ding J, Liao C, Xu J, Liu X, Lu W. Defensins: The natural peptide antibiotic. Adv Drug Deliv Rev. 2021;179:114008. https://doi.org/10.1016/j.addr.2021.114008.
23. Du Y, Yang Y, Zhang W, Yang C, Xu P. Human β-defensin-3 and nuclear factor-kappa B p65 synergistically promote the cell proliferation and invasion of oral squamous cell carcinoma. Transl Oncol. 2023;27: 1-7. https://doi.org/10.1016/j.tranon.2022.101582.
24. Ghosh SK, McCormick TS, Weinberg A. Human beta defensins and cancer: contradictions and common ground. Front Oncol. 2019;9: 1-8. https://doi.org/10.3389/fonc.2019.00341.
25. Zhang L. Different dynamics and pathway of disulfide bonds reduction of two human defensins, a molecular dynamics simulation study. Proteins Struct Funct Bioinforma. 2017;85(4):665–81. https://doi.org/10.1002/prot.25247.
26. Mohammed NUG, Gorial FI, Khaleel FM, Abed BA, Mutar SA, Farhan LO, et al. Role of Human β-Defensin-3 in Rheumatoid Arthritis: An Observational Single-Center Study. Al-Rafidain J Med Sci (ISSN 2789-3219). 2023;5(1S):S71-75. https://doi.org/10.54133/ajms.v5i1S.289.
27.ABED E, Rasheed MK, Hussein KG. Assessment of Total Procollagen Type 1 Intact N-terminal Propeptide, C-telopeptide of type 1 collagen, Bone Mineral Density and its Relationship to Body Mass Index in Men with Type 2 Diabetes. J Fac Med Baghdad. 2022;64(2):81–5. https://doi.org/10.32007/jfacmedbagdad.6421942.
28. Gürsoy UK, Salli K, Söderling E, Gürsoy M, Hirvonen J, Ouwehand AC. Regulation of hBD-2, hBD-3, hCAP18/LL37, and proinflammatory cytokine secretion by human milk oligosaccharides in an organotypic oral mucosal model. Pathogens. 2021;10(6): 1-7. https://doi.org/10.3390/pathogens10060739.
29. Liang W, Diana J. The dual role of antimicrobial peptides in autoimmunity. Front Immunol. 2020;11: 1-9. https://doi.org/10.3389/fimmu.2020.02077.
30. Para R, Romero R, Miller D, Panaitescu B, Varrey A, Chaiworapongsa T, et al. Human β-defensin-3 participates in intra-amniotic host defense in women with labor at term, spontaneous preterm labor and intact membranes, and preterm prelabor rupture of membranes. J Matern Neonatal Med. 2020;33(24):4117–32. https://doi.org/10.1080/14767058.2019.1597047.
31. Peng G, Tsukamoto S, Ikutama R, Nguyen HLT, Umehara Y, Trujillo-Paez J V, et al. Human β-defensin-3 attenuates atopic dermatitis–like inflammation through autophagy activation and the aryl hydrocarbon receptor signaling pathway. J Clin Invest. 2022;132(17) : 1-16. https://doi.org/10.1172/JCI156501.
32. Li L, Jiang H, Chen R, Zhou J, Xiao Y, Zhang Y, et al. Human β-defensin 3 gene modification promotes the osteogenic differentiation of human periodontal ligament cells and bone repair in periodontitis. Int J Oral Sci. 2020;12(1):13. https://doi.org/10.1038/s41368-020-0078-6.
33. Park OJ, Kim J, Ahn KB, Lee JY, Park YJ, Kum KY, et al. A 15-amino acid C-terminal peptide of beta-defensin-3 inhibits bone resorption by inhibiting the osteoclast differentiation and disrupting podosome belt formation. J Mol Med [Internet]. 2017;95(12):1315–25. https://doi.org/10.1007/s00109-017-1589-2.
34. Gao X, Feng J, Wei L, Dong P, Chen J, Zhang L, et al. Defensins: A novel weapon against Mycobacterium tuberculosis? Int Immunopharmacol. 2024;127:111383. https://doi.org/10.1016/j.intimp.2023.111383.
35.Ahmed AH. Possible relationships of selected food items to osteoporosis among a group of Iraqi women. J Fac Med Baghdad. 2021;63(4):171–5. https://doi.org/10.32007/jfacmedbagdad.6341868.
36. Jafer AA, Ali BH. Evaluation of Osteocalcin and Some Biochemical Marker in Diabetes Mellitus Iraqi Women’s patients with Osteoporosis. Ibn AL-Haitham J Pure Appl Sci. 2023;36(1):225–35. https://doi.org/10.30526/36.1.2984.
37. Farhan LO, Taha EM, Farhan AM. A Case control study to determine Macrophage migration inhibitor, and N-telopeptides of type I bone collagen Levels in the sera of osteoporosis patients. Baghdad Sci J. 2022;19(4):848-54. http://dx.doi.org/10.21123/bsj.2022.19.4.0848.
38.Abdlkarem HA, Zainulabdeen JA. A Comparative Study of Vitamin D Level and Lactate Dehydrogenase Activity in Relation to Oxidative Stress in Women with Osteoporosis. J Fac Med Baghdad. 2024;66(1):110–5. https://doi.org/10.32007/jfacmedbagdad.6612255.
39. Polzonetti V, Pucciarelli S, Vincenzetti S, Polidori P. Dietary Intake of Vitamin D from Dairy Products Reduces the Risk of Osteoporosis. Nutrients [Internet]. 2020 [cited 2023 May 15]; 12 (6): 1743. https://doi.org/10.3390/nu12061743.
40. Tuckey RC, Cheng CYS, Slominski AT. The serum vitamin D metabolome: What we know and what is still to discover. J Steroid Biochem Mol Biol. 2019;186:4–21. https://doi.org/10.1016/j.jsbmb.2018.09.003.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Zahraa S. Hassan, Layla O. Farhan

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


