Long-Term Effects of Scopolamine on Brain Tissue of Mice
DOI:
https://doi.org/10.32007/jfacmedbaghdad.6632323Keywords:
Alzheimer’s disease, Antioxidant, Cognitive function, Oxidative stress, ScopolamineAbstract
Background: Scopolamine is an anticholinergic drug that disrupts cholinergic transmission in the central nervous system as well as causes cognitive abnormalities and pathological hallmarks that are similar to those seen in Alzheimer’s Disease. Therefore, it is used for induction of Alzheimer’s Disease in animal models.
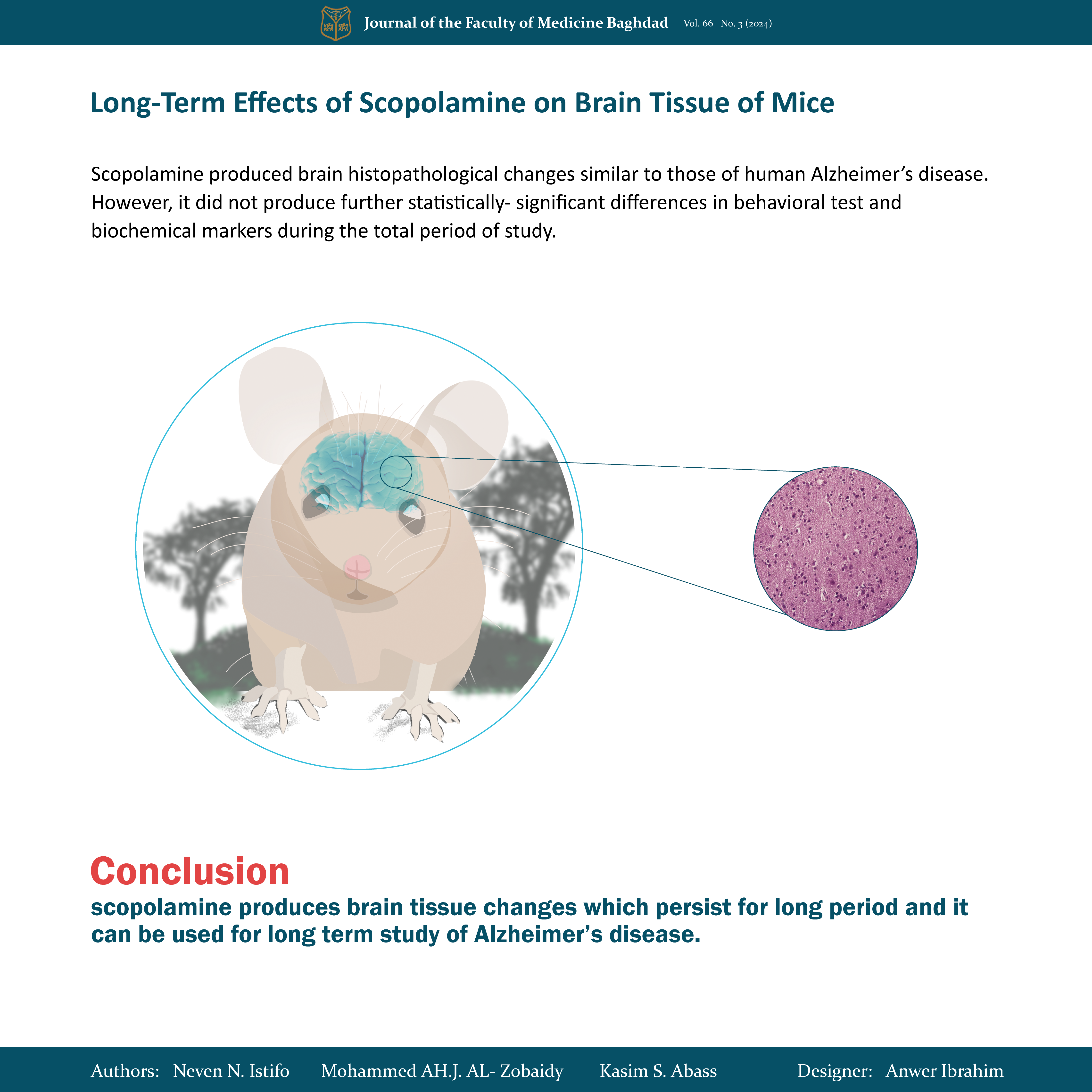
Objective: to investigate the effects of long-term induction with scopolamine on the brain tissue of mice.
Methods: Seventy adult mice were divided into 2 equal groups: The first group was the normal control group received distilled water only. The second one was the Alzheimer’s Disease induction group received intraperitoneal scopolamine (1mg/kg) for 14 days only after that distilled water was given for the next 6 months. Ten mice were isolated from each group at zero time, after 2 weeks of induction, after 3-month and after 6 months and subjected to the behavioral tests then sacrificed for determination of biochemical factors (including brain-derived neurotrophic factor, total antioxidant status, malondialdehyde, and amyloid β). Data were analyzed using t-tests, and ANOVA. All values expressed as Mean±SD and P value <0.05 were considered significant.
Result: Scopolamine produced brain histopathological changes similar to those of human Alzheimer’s disease. However, it does not produce further statistically significant differences in behavioral tests and biochemical markers during the total period of study.
Conclusion: scopolamine produces brain tissue changes that persist for a long period and it can be used for long-term study of Alzheimer’s disease.
Received: Feb., 2024
Revised: May 2024
Accepted: July 2024
Downloads
References
Cheon SY, Koo B-N, Kim SY, Kam EH, Nam J, Kim EJ. Scopolamine promotes neuroinflammation and delirium-like neuropsychiatric disorder in mice. Scientific Reports. 2021;11(1):8376.
https://doi.org/10.1038/s41598-021-87790-y.
Chen WN, Yeong KY. Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2020;19(2):85-93. https://doi.org/10.2174/1871527319666200214104331.
Yadang FSA, Nguezeye Y, Kom CW, Betote PHD, Mamat A, Tchokouaha LRY, et al. Scopolamine-induced memory impairment in mice: neuroprotective effects of Carissa edulis (Forssk.) Valh (Apocynaceae) aqueous extract. International Journal of Alzheimer’s Disease. 2020;2020. https://doi.org/10.1155/2020/6372059.
Rahimzadegan M, Soodi M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic and clinical neuroscience. 2018;9(1):5. https://doi.org/10.29252/NIRP.BCN.9.1.5.
Hernández-Rodríguez M, Arciniega-Martínez IM, García-Marín ID, Correa-Basurto J, Rosales-Hernández MC. Chronic administration of scopolamine increased GSK3βP9, beta secretase, amyloid beta, and oxidative stress in the hippocampus of Wistar rats. Molecular Neurobiology. 2020;57:3979-88. https://doi.org/10.1007/s12035-020-02009-x.
Mahdi O, Baharuldin MTH, Nor NHM, Chiroma SM, Jagadeesan S, Moklas MAM. Chemicals used for the induction of Alzheimer’ s disease-like cognitive dysfunctions in rodents. Biomedical Research and Therapy. 2019;6(11):3460-84. https://doi.org/10.15419/bmrat.v6i11.575.
Pitts MW. Barnes maze procedure for spatial learning and memory in mice. Bio-protocol. 2018;8(5):e2744-e. https://doi.org/10.21769/BioProtoc.2744.
Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. JoVE (Journal of Visualized Experiments). 2017(126):e55718. https://doi.org/10.3791/55718.
Prieur EA, Jadavji NM. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio-protocol. 2019; 9 (3): e3162-ed https://doi.org/10.21769/BioProtoc.3162.
Kirkwood BR, Sterne JA. Essential medical statistics: John Wiley & Sons; 2010.
DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Molecular neurodegeneration. 2019;14(1):1-18. https://doi.org/10.1186/s13024-019-0333-5.
Miller JA, Horvath S, Geschwind DH. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proceedings of the National Academy of Sciences. 2010;107(28):12698-703. https://doi.org/10.1073/pnas.0914257107
Radulescu CI, Cerar V, Haslehurst P, Kopanitsa M, Barnes SJ. The aging mouse brain: cognition, connectivity and calcium. Cell Calcium. 2021;94:102358. https://doi.org/10.1016/j.ceca.2021.102358.
Shoji H, Miyakawa T. Age‐related behavioral changes from young to old age in male mice of a C57 BL/6J strain maintained under a genetic stability program. Neuropsychopharmacology reports. 2019;39(2):100-18. https://doi.org/10.1002/npr2.12052
Hendrickx JO, De Moudt S, Calus E, De Deyn PP, Van Dam D, De Meyer GR. Age-related cognitive decline in spatial learning and memory of C57BL/6J mice. Behavioural brain research. 2022; 418: 113649. https://doi.org/10.1016/j.bbr.2021.113649.
Crespo NE. Age-Related Cognitive Decline in Female C57BL/6cenp Mice 15-16 Months of Age. J Biomed Eng. 2021; 5: 1-12. https://doi.org/10.17303/jber.2021.5.101.
Clifford KP, Miles AE, Prevot TD, Misquitta KA, Ellegood J, Lerch JP, et al. Brain structure and working memory adaptations associated with maturation and aging in mice. Frontiers in Aging Neuroscience. 2023; 15. https://doi.org/10.3389/fnagi.2023.1195748.
Chi H, Chang H-Y, Sang T-K. Neuronal cell death mechanisms in major neurodegenerative diseases. International journal of molecular sciences. 2018; 19 (10): 3082. https://doi.org/10.3390/ijms19103082.
Janssen L, Keppens C, De Deyn PP, Van Dam D. Late age increase in soluble amyloid-beta levels in the APP23 mouse model despite steady-state levels of amyloid-beta-producing proteins. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2016;1862(1):105-12. https://doi.org/10.1016/j.bbadis.2015.10.027.
Ameen-Ali KE, Simpson JE, Wharton SB, Heath PR, Sharp PS, Brezzo G, et al. The time course of recognition memory impairment and glial pathology in the hAPP-J20 mouse model of Alzheimer’s disease. Journal of Alzheimer's Disease. 2019;68(2):609-24.https://doi.org/10.3233/JAD181238.
Endres T, Lessmann V. Age-dependent deficits in fear learning in heterozygous BDNF knock-out mice. Learning & memory. 2012;19(12):561-70. http://www.learnmem.org/cgi/doi/10.1101/lm.028068.112.
Psotta L, Lessmann V, Endres T. Impaired fear extinction learning in adult heterozygous BDNF knock-out mice. Neurobiology of learning and memory. 2013;103:34-8.
https://doi.org/10.1016/j.nlm.2013.03.003
Harb M, Jagusch J, Durairaja A, Endres T, Leßmann V, Fendt M. BDNF haploinsufficiency induces behavioral endophenotypes of schizophrenia in male mice that are rescued by enriched environment. Translational Psychiatry. 2021;11(1):233. https://doi.org/10.1038/s41398-021-01365-z.
Cases S, Saavedra A, Tyebji S, Giralt A, Alberch J, Pérez-Navarro E. Age-related changes in STriatal-Enriched protein tyrosine Phosphatase levels: Regulation by BDNF. Molecular and Cellular Neuroscience. 2018;86:41-9. https://doi.org/10.1016/j.mcn.2017.11.003.
Walker MP, LaFerla FM, Oddo SS, Brewer GJ. Reversible epigenetic histone modifications and Bdnf expression in neurons with aging and from a mouse model of Alzheimer’s disease. Age. 2013;35:519-31. https://doi.org/10.1007/s11357-011-9375-5.
Ceballos-Picot I, Nicole A, Clément M, Bourre J-M, Sinet P-M. Age-related changes in antioxidant enzymes and lipid peroxidation in brains of control and transgenic mice overexpressing copper-zinc superoxide dismutase. Mutation Research/DNAging. 1992;275(3-6):281-93. https://doi.org/10.1016/0921-8734(92)90032-k.
Leyane TS, Jere SW, Houreld NN. Oxidative stress in ageing and chronic degenerative pathologies: molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. International journal of molecular sciences. 2022;23(13):7273. https://doi.org/10.3390/ijms23137273.
Heurtaux T, Bouvier DS, Benani A, Helgueta Romero S, Frauenknecht KB, Mittelbronn M, et al. Normal and pathological NRF2 signalling in the central nervous system. Antioxidants. 2022;11(8):1426. https://doi.org/10.3390/antiox11081426 .
Illes P, Rubini P, Ulrich H, Zhao Y, Tang Y. Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells. 2020;9(5):1108. https://doi.org/10.3390/cells9051108.
Woo Y, Lim JS, Oh J, Lee JS, Kim J-S. Neuroprotective effects of euonymus alatus extract on scopolamine-induced memory deficits in mice. Antioxidants. 2020; 9 (5) :449. https://doi.org/10.3390/antiox9050449.
Cheedella HK, Silakabattini K, Siahmansur TJ, Ishaq BM. Evaluation Of Neuroprotective Activity In Scopolamine Induced Dementia In Wistar Rats By Using Various Pharmacological Equipment And Its Histopathology. Journal of Survey in Fisheries Sciences. 2023: 1299-307. https://sifisheriessciences.com/index.php/journal/article/view/813/363.
Lee JC, Park JH, Ahn JH, Park J, Kim IH, Cho JH, et al. Effects of chronic scopolamine treatment on cognitive impairment and neurofilament expression in the mouse hippocampus. Molecular medicine reports. 2018;17(1):1625-32. https://doi.org/10.3892/mmr.2017.8082
Kim JH, Han Y-E, Oh S-J, Lee B, Kwon O, Choi CW, et al. Enhanced neuronal activity by suffruticosol A extracted from Paeonia lactiflora via partly BDNF signaling in scopolamine-induced memory-impaired mice. Scientific Reports. 2023;13(1):11731. https://doi.org/10.1038/s41598-023-38773-8.
Ban JY, Park HK, Kim SK. Effect of glycyrrhizic acid on scopolamine-induced cognitive impairment in mice. International Neurourology Journal. 2020;24(Suppl 1):S48. https://doi.org/10.5213/inj.2040154.077.
Bae HJ, Sowndhararajan K, Park H-B, Kim S-Y, Kim S, Kim DH, et al. Danshensu attenuates scopolamine and amyloid-β-induced cognitive impairments through the activation of PKA-CREB signaling in mice. Neurochemistry International. 2019;131:104537. https://doi.org/10.1016/j.neuint.2019.104537.
Aykac A, Ozbeyli D, Uncu M, Ertaş B, Kılınc O, Şen A, et al. Evaluation of the protective effect of Myrtus communis in scopolamine-induced Alzheimer model through cholinergic receptors. Gene. 2019; 689:194-201. https://doi.org/10.1016/j.gene.2018.12.007.
Anand A, Khurana N, Ali N, AlAsmari AF, Alharbi M, Waseem M, et al. Ameliorative effect of vanillin on scopolamine-induced dementia-like cognitive impairment in a mouse model. Frontiers in Neuroscience. 2022;16:1005972. https://doi.otg/10.3389/fnins.2022.1005972.
Lee M-R, Yun B-S, Park S-Y, Ly S-Y, Kim S-N, Han B-H, et al. Anti-amnesic effect of Chong–Myung–Tang on scopolamine-induced memory impairments in mice. Journal of ethnopharmacology. 2010;132(1):70-4. https://doi.org/10.1016/j.jep.2010.07.041
Sharma C, Kim SR. Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants. 2021;10(8):1231. https://doi.org/10.3390/antiox10081231
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Neven N. Istifo, Mohammed A. AL- Zobaidy, Kasim S. Abass

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


