Effect of Salivary Melatonin on Ionizing Radiation Worker and its Effect on Periodontal Disease
DOI:
https://doi.org/10.32007/jfacmedbagdad.6612183Keywords:
Antioxidant, Melatonin, Periodontal disease, Radiation, Reactive oxygen speciesAbstract
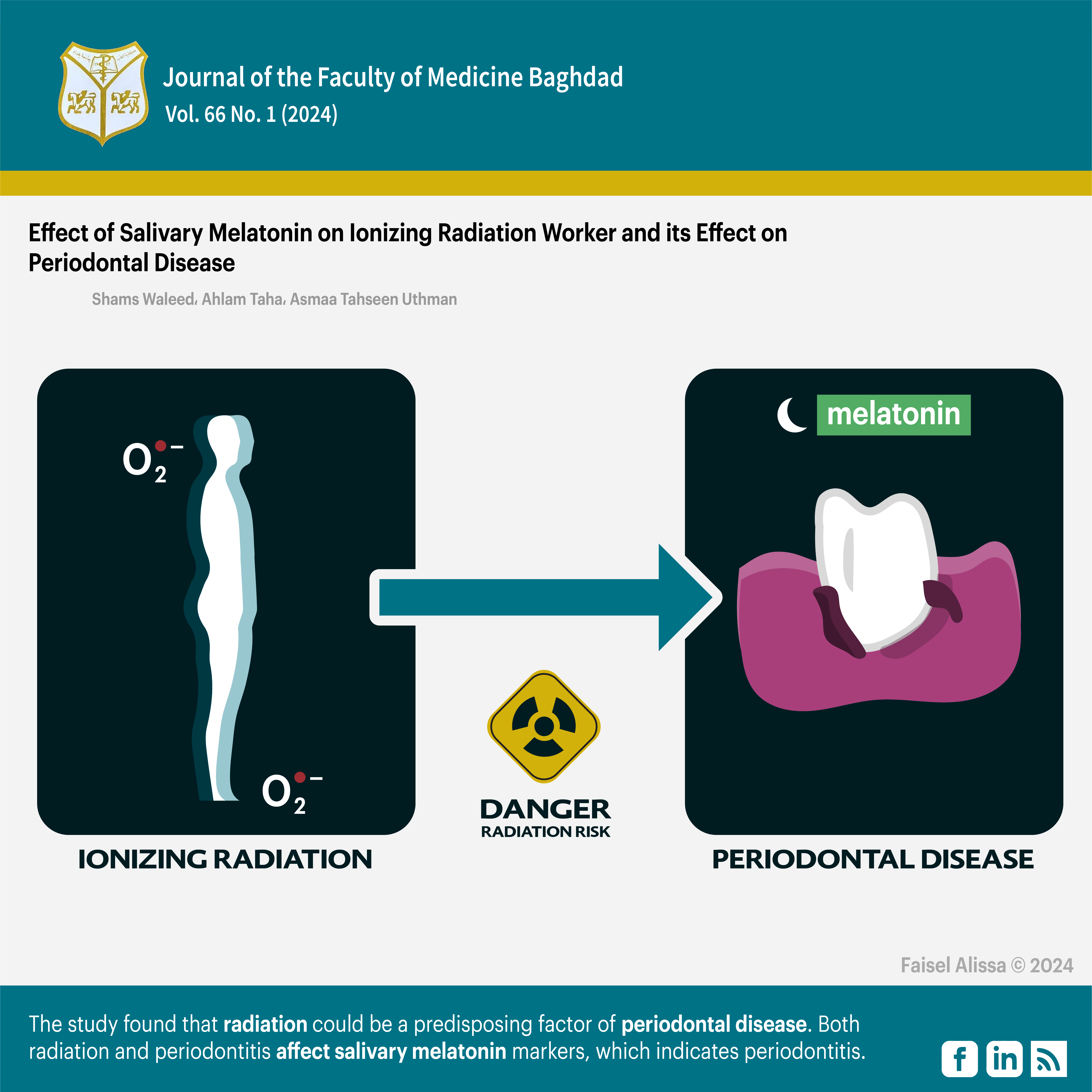
Background: The use of ionizing radiation, such as radiotherapy and medical imaging, is common in modern medicine. However, it can have negative effects on the body, such as an imbalance in oxidative stress, which can lead to periodontal or gum disease. Radiographers are particularly at risk of experiencing this due to increased levels of reactive oxygen species. Antioxidant therapy, such as melatonin, may help to minimize oxidative stress and protect tissues.
Objectives: The this study aimed to evaluate the impact of radiation on periodontal conditions among radiation workers, as well as to assess the levels of melatonin in their bodies.
Patients and Methods: The study group consisted of 40 men who had worked with ionizing radiation for 5 years or more, while the control group consisted of 40 men who worked as nurses or lab workers. Unstimulated salivary samples were collected from all subjects to measure salivary melatonin levels. Both groups were then evaluated for the plaque index, bleeding on probing, probing pocket depth, and clinical attachment loss.
Results: The results of the study showed that the study group had a higher mean plaque index (1.41 ± 0.484) compared to the control group (1.14±0.524) (p=0.013), a higher mean probing pocket depth (4.40±1.463) compared to the control group (2.82±2.328) (P=0.0002), and a higher mean clinical attachments loss (2.67 ±1.461) compared to the control group (1.59±1.919) (P=0.004). However, there was no significant difference in the mean gingival bleeding between the two groups. Additionally, the study group had a lower mean salivary melatonin level (34.19±13.849) compared to the control group (42.43±16.783) (P=0.013).
Conclusion: This study suggests that radiation exposure may increase the risk of periodontal disease. The results also indicate that both radiation and periodontitis can affect salivary melatonin markers, which may be useful in identifying periodontitis.
Received: Jul,, 2023
Accepted: Oct, 2023
Published: Aprl.2024
Downloads
References
Hickling S, Xiang L, Jones KC, Parodi, K, Assman, W. Avery S, et al. Ionizing radiation-induced acoustics for radiotherapy and diagnostic radiology applications. Med. Phys. 2018, 45, 707–721. https://doi.org/10.1002/mp.12929.
Buonanno M, de Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat. Res. 2011, 175, 405–415. https://doi.org/10.1667/RR2461.1.
Kirsch DG, Diehn M, Kesarwala AH, Maity A, Morgan MA, Schwarz JK, et al. The Future of Radiobiology. J. Natl. Cancer Inst. 2018, 110, 329–340. https://doi.org/10.1093/jnci/djx231.
Cui F, Ma N, Han X, Chen N, Xi Y, Yuan W, et al. Effects of 60 Co γ gamma Irradiation on the Reproductive Function of Caenorhabditis elegans. Dose-Response 2019, 17, 1–6. https://doi.org/10.1177/1559325818820981.
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, et al. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. https://doi.org/10.2174%2F1570159X14666161228122115
Cipolla-Neto J, Amaral FGD. Melatonin as a Hormone: New Physiological and Clinical
Insights. Endocr. Rev. 2018, 39, 990–1028. https://doi.org/10.1210/er.2018-00084.
Mortezaee K, Potes Y, Mirtavoos-Mahyari H, Motevaseli E, Shabeeb D, Musa AE, et al. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 1–8. https://doi.org/10.1016/j.lfs.2019.116960
Hardeland R. Aging, Melatonin, and the Pro-and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. https://doi.org/10.3390/ijms20051223
Zhang HM, Zhang Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. https://doi.org/10.1111/jpi.12162.
Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. https://doi.org/10.1016/j.lfs.2017.02.008
Eke PI, Zhang X, Lu H, Wei L, Thornton-Evans G. Greenlund KJ, et al. Predicting Periodontitis at State and Local Levels in the United States. J. Dent. Res. 2016, 95, 515–522. https://doi.org/10.1177/0022034516629112.
Isola G, Polizzi A, Alibrandi A, Williams RC, Leonardi R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020, https://doi.org/10.1002/JPER.20-0242.
Correa MG, Absy S, Tenenbaum H, Ribeiro FV, Cirano FR, Casati MZ, et al. Resveratrol attenuates oxidative stress during experimental periodontitis in rats exposed to cigarette smoke inhalation. J. Periodontal. Res. 2019, 54, 225–232. https://doi.org/10.1111/jre.12622
Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial‐mesenchymal transition. Journal of periodontal research. 2018 Aug;53(4):565-74.. https://doi.org/10.1111/jre.12546.
Khaudyer AT, Mohammed AT and Al-Ghurabi BH. Oral health condition among kindergarten children in Relation to Salivary soluble Cluster of Differentiation 14 and Tool Like Receptor 2 (comparative study). Indian Journal of Public Health, 2019,10(9), p.1407. http://dx.doi.org/10.5958/0976-5506.2019.02643.3.
Hasan RS. Evaluation of Salivary Melatonin, Secretory IgA Levels and Periodontal Parameters in Type 2 Diabetic Patients with Chronic Periodontitis (A comparative study) (Doctoral dissertation, council of the College of Dentistry, University of Baghdad). 2019. http://dx.doi.org/10.5958/0976-5506.2019.03283.2
Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. https://doi.org/10.1111/jcpe.13112
Alhussainy GN, Mohammed AT, and Hussein B. "Salivary Oxidative Status in Relation to Periodontal Status among Workers in Diagnostic Radiation Field." International Journal of Medical Research & Health Sciences 2018 ,7, no. 9 66-71. http://dx.doi.org/10.1111/j.1601-5037.2005.00125.x
Kamil TF and Ali OH. Association between The Cytokine IL-23 in Saliva with Periodontal Health and Disease. The Egyptian Journal of Hospital Medicine, 2023. 91(1), pp.3920-3924. https://doi.org/10.21608/ejhm.2023.294152.
Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontology 2000. 2013 Jun; 62(1):59-94. https://doi.org/10.1111/j.1600-0757.2012.00457.x
Sadetzki S, Chetrit A, Harold DSC, Mann J, Amitai T, Even-Nir H et al. Long-term effects of exposure to ionizing irradiation on periodontal health status – the Tinea capitis cohort study; Front., Public Health 2014. 3:226.
https://doi.org/10.3389%2Ffpubh.2015.00226
Sallam RA. Characteristics of oral condition among workers in the field of radiation. Characteristics of oral condition among workers in the field of radiation. 2016 PHD thesis. https://www.researchgate.net/publication/234064531_Study_of_oral_health_among_workers_in_the_field_of_radiation
Syrjala A, Raatikainen L, Komulainen K, Knuuttila M, Ruoppi P, Hartikainen S, et al. Salivary flow rate and periodontal infection: a study among subjects aged 75 years or older.2011, Oral Diseases, 17, 387-392. https://doi.org/10.1111/j.1601-0825.2010.01764.x.
Ahmad IM, Temme JB, Abdalla MY, Zimmerman MC. Redox Status in Workers Occupationally Exposed to Long Term Low Levels of Ionizing Radiation -A pilot study; Redox Rep.2016, May; 21(3): 139–145 https://doi.org/10.1080/13510002.2015.1101891.
Srinath R, Acharya AB, Thakur SL. Salivary and gingival crevicular fluid melatonin in periodontal health and disease. J Periodontol. 2010 Feb;81(2):277-83. https://Doi.org/10.1902/jop.2009.090327.
Almughrabi O, Marzouk K, Hasanato R, Shafik S. and SHAFIK, S. Melatonin levels in periodontal health and disease. Journal of periodontal research.2013, 48, 315-321. https://doi.org/10.1902/jop.2009.090327
Ghallab NA, Hamdy E, Shaker OG. Malondialdehyde, superoxide dismutase and melatonin levels in gingival crevicular fluid of aggressive and chronic periodontitis patients. Aust Dent J. 2016 Mar;61(1):53-61. https://doi.org/10.1111/adj.12294
Lodhi K, Saimbi CS, Khan MA, Nath C and Shukla R. Evaluation of melatonin levels in saliva in gingivitis and periodontitis cases: A pilot study. 2016, Contemporary clinical dentistry, 7, 519. https://doi.org/10.4103%2F0976-237X.194115
Abdolsamadi H, Goodarzi MT, Ahmadi Motemayel F, Jazaeri M, Feradmal J, Zarabadi M, Hoseyni M, Torkzaban P. Reduction of Melatonin Level in Patients with Type II Diabetes and Periodontal Diseases. J Dent Res Dent Clin Dent Prospects. 2014 Summer;8(3):160-5. https://Doi.org/10.5681/joddd.2014.029
Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, Rezapoor S. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017 Apr;9(2):139-148. https://Doi.org/10.1007/s12551-017-0256-8
Szczepanik M. Melatonin and its influence on immune system. J Physiol Pharmacol. 2007 Dec;58 Suppl 6:115-24.. https://doi.org/10.3390/ijms14048638
Mrnka L, Hock M, Rybová M, Pácha J. Melatonin inhibits prostaglandin E2-and sodium nitroprusside-induced ion secretion in rat distal colon. Eur J Pharmacol. 2008 Feb26;581(1-2):164-70 https://doi.org/10.1016/j.ejphar.2007.11.031
Viswanathan M. Melatonin inhibits calcitonin gene-related peptide-induced vasodilation and increase in cAMP in rat middle cerebral arteries., European journal of pharmacology.2001, 415, 247-250. https://doi.org/10.1016/S0014-2999(01)00826-3
Satomura K, Tobiume S, Tokuyama R, Yamasaki Y, Kudoh K, Maeda E, et al. Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. Journal of pineal research.2007, 42, 231-239. https://doi.org/10.1111/j.1600-079X.2006.00410.x
Zaminy A, Ragerdi Kashani I, Barbarestani M, Hedayatpour A, Mahmoudi R, Farzaneh Nejad A. Osteogenic differentiation of rat mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells: melatonin as a differentiation factor. Iran Biomed J. 2008 Jul;12(3):133-41. https://doi.org/10.1590/1678-4324-2016150383
Cutando A, Gómez-Moreno G, Arana C, Acuñacastroviejo D, Reiter RJ. Melatonin: potential functions in the oral cavity. Journal of periodontology.2012, 78, 1094-1102. https://doi.org/10.1902/jop.2007.060396
Kara A, Akman S, Ozkanlar S, Tozoglu U, Kalkan Y, Canakci CF, Tozoglu S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic Biol Med. 2013 Feb;55:21-6. https://doi.org/10.1016/j.freeradbiomed.2012.11.002
Ismail HS, Mahmood MS. Effect of melatonin supplementation on the gingival health and lipid profiles in obese periodontitis patients. Journal of Baghdad College of Dentistry. 2022 Mar 15;34(1):51-9. https://doi.org/10.26477/jbcd.v34i1.3092
Saliem SS, Bede SY, Cooper PR, Abdulkareem AA, Milward MR and Abdullah, BH. 2022. Pathogenesis of periodontitis–A potential role for epithelial-mesenchymal transition. Japanese Dental Science Review, 58, pp.268-278. http://dx.doi.org/10.1016/j.jdsr.2022.09.001
Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. The Journal of the American Dental Association. 2008; 139: 35S- 40S. https://doi.orgl10.14219/jada.archive.2008.0353. 41. Carvalho SPM, Sales-Peres A, Ribeiro-Bicudo LA, Silva RHAd. Quality evaluation of DNA obtained from stored human saliva and its applicability to identification in Forensic Dentistry. Revista Odonto Ciência. 2010;25:48- http://dx.doi.org/10.1590/S1980-65232010000100010
Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2024 Shams Waleed, Ahlam taha , Asmaa Tahseen Uthman

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


