Characterization of Gram-positive Bacteria Isolated from Gallstones of Iraqi Patients
DOI:
https://doi.org/10.32007/jfacmedbaghdad3209Keywords:
Biofilm, Cholesterol, Enterococcus, Gallstone disease, Staphylococcus aureusAbstract
Background: Gallstone disease is a major global health problem, the prevalence of which depends on metabolic, genetic, and infectious factors. Recent reports point out that Gram-positive bacteria, mainly Staphylococcus aureus and Enterococcus species, are also involved in the development of gallstones via enzyme action and biofilm formation.
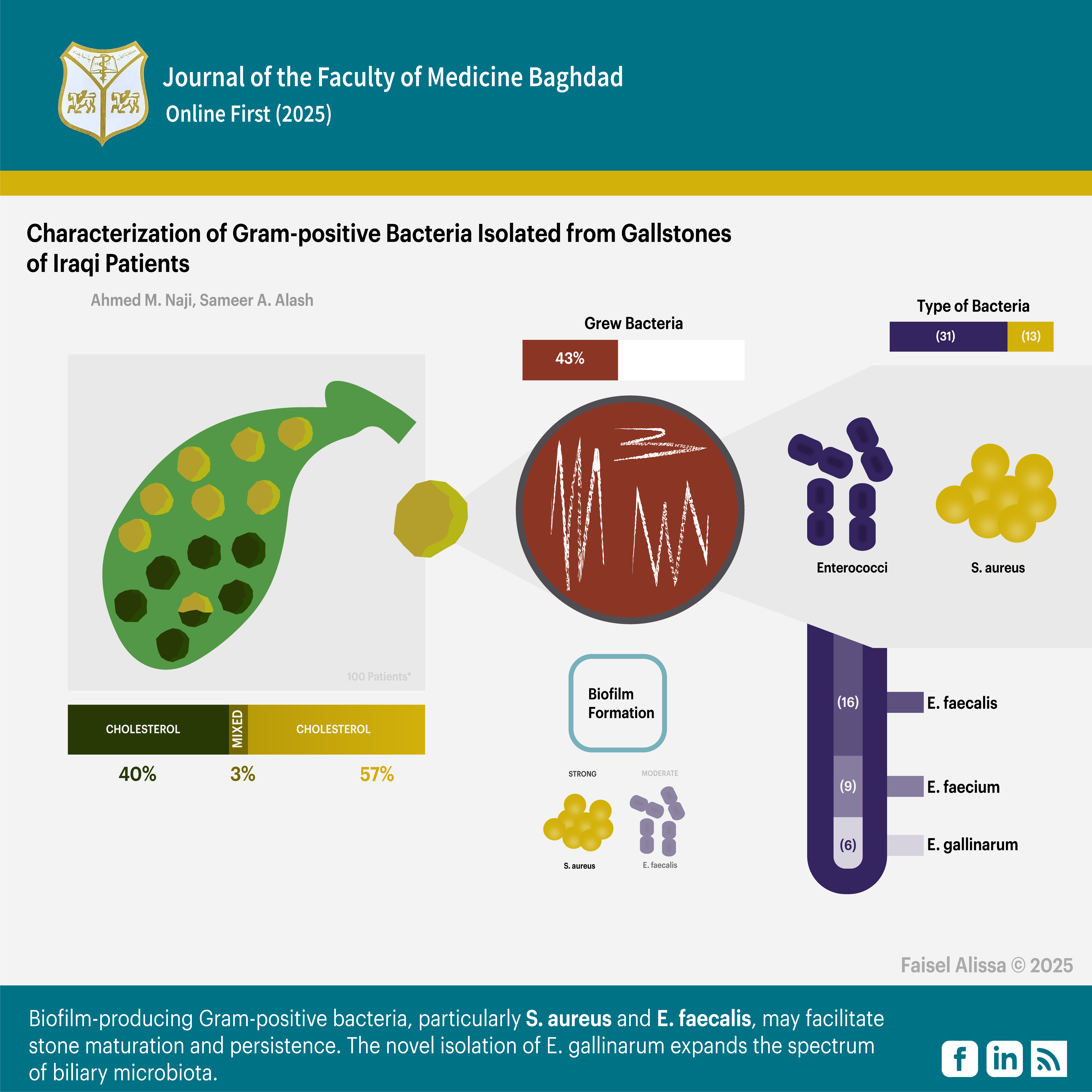
Objectives: To characterize Gram-positive bacteria within gallstones from Iraqi patients, evaluate their biofilm-forming capacity, and analyze the relationship between bacterial colonization, gallstone type, as well as cholesterol levels.
Methods: A total of 100 gallstones were obtained from 100 patients undergoing elective cholecystectomy between October 2024 and March 2025. Stones were aseptically processed for bacterial isolation and identification using selective culture media and the VITEK® 2 Compact System. Serum cholesterol levels were determined by enzymatic colorimetric assay. Biofilm formation was quantified via the 96-well microtiter plate method, and statistical correlations between gallstone type, cholesterol level, and bacterial presence were analyzed. Chi-square assessed the association between gallstone type and bacterial count, while Mann-Whitney U and Kruskal-Wallis tested differences in study variables.
Results: Cholesterol stones were more prevalent than pigment and mixed stones (57%, 40%, 3%), respectively. Bacterial growth was observed in 43% of gallstones, with Enterococcus species (31 isolates) predominating over S. aureus (12 isolates). Species-level identification revealed E. faecalis (n= 16), E. faecium (n= 9), and E. gallinarum (n= 6), marking the first reported isolation of E. gallinarum from gallstones. Cholesterol concentrations were significantly higher in sterile stones (median 235 mg/dl) compared to bacteria-positive stones (173-186 mg/dl). Biofilm analysis showed all S. aureus isolates as strong producers, whereas E. faecalis exhibited predominantly moderate-to-strong formation, while E. faecium and E. gallinarum displayed weaker capacities.
Conclusion: There is a significant interplay between microbial colonization and gallstone composition. Strong biofilm-producing Gram-positive bacteria, particularly S. aureus and E. faecalis, may facilitate stone maturation and persistence. The novel isolation of E. gallinarum expands the spectrum of biliary microbiota.
Received: Sept. 2025
Revised: Dec. 2025
Accepted: Dec. 2025
Published Online: Dec. 2025
Published: Dec. 2025
Downloads
References
1.Jumaa AM, Hussein AL, Khalaf MA. Gallstones in Tikrit, Iraq: A population-based study on the roles of obesity, gender, and age. In: An Overview of Disease and Health Research, Vol. 1. BP International; 2025. p. 1-10. https://doi.org/10.9734/bpi/aodhr/v1/5254 DOI: https://doi.org/10.9734/bpi/aodhr/v1/5254
2.Mohammed AM, Mohammed AM, Hassan TY. Prevalence of Cholelithiasis and Associated Factors of Gallstone Formation after Laparoscopic Sleeve Gastrectomy in the Gastroenterology and Hepatology Teaching Hospital-Baghdad. Journal of the Faculty of Medicine Baghdad. 2024;66(4):473-8. https://doi.org/10.32007/jfacmedbaghdad.6642381 DOI: https://doi.org/10.32007/jfacmedbaghdad.6642381
3. Taha EM, Mohialdeen Taha M, Al-Obaidy SK, Faris Hasan B, Rahim SM. Association between atherogenic index and cholesterol to HDL ratio in COVID-19 patients during the initial phase of infection. Archives of Razi Institute. 2022;77(3):1311.
https://doi.org/10.22092/ARI.2022.357527.2057
4.Mazyad MS, Abdul-Ghafoor BH. Study the predicators and risk factors for formation of gallstones in a sample of asymptomatic Iraqi patients in Baghdad. International Surgery Journal. 2023;10(5):829-36. https://doi.org/10.18203/2349-2902.isj20231056 DOI: https://doi.org/10.18203/2349-2902.isj20231056
5.Sun H, Warren J, Yip J, et al. Factors influencing gallstone formation: a review of the literature. Biomolecules. 2022;12(4):550. https://doi.org/10.3390/biom12040550 DOI: https://doi.org/10.3390/biom12040550
6.Komorniak N, Pawlus J, Gawel K, Hawryłkowicz V, Stachowska E. Cholelithiasis, Gut Microbiota and Bile Acids after Bariatric Surgery- Can Cholelithiasis Be Prevented by Modulating the Microbiota? A Literature Review. Nutrients. 2024;16(15):2551.
https://doi.org/10.3390/nu16152551 DOI: https://doi.org/10.3390/nu16152551
7.Abdullah BH, Jassam SA, Hadi WA, Hameed B. Gallbladder stone formation in Iraqi patients is associated with bacterial infection and HLA class II-DRB1 antigens. Indian Journal of Pathology and Microbiology. 2020;63(4):570-4.
https://doi.org/10.4103/IJPM.IJPM_841_19 DOI: https://doi.org/10.4103/IJPM.IJPM_841_19
8.Xie M, Zhang X, Wu Y, et al. Alterations of biliary and gut microbiota in relation to gallstone formation. Journal of Clinical and Translational Research. 2024;10(3):180-90. https://doi.org/10.36922/jctr.23.00118 DOI: https://doi.org/10.36922/jctr.23.00118
9.Banerjee T, Goswami AG, Basu S. Biliary microbiome and gallstones: A silent friendship. World Journal of Gastrointestinal Surgery. 2024;16(11):3395-9. https://doi.org/10.4240/wjgs.v16.i11.3395 DOI: https://doi.org/10.4240/wjgs.v16.i11.3395
10.Xiao M, Zhou Y, Wang Z, et al. The dysregulation of biliary tract microflora is closely related to primary choledocholithiasis: a multicenter study. Scientific Reports. 2024; 14:9004. https://doi.org/10.1038/s41598-024-59737-6 DOI: https://doi.org/10.1038/s41598-024-59737-6
11.Yang XT, Wang J, Jiang YH, et al. Insight into the mechanism of gallstone disease by proteomic and metaproteomic characterization of human bile. Frontiers in Microbiology. 2023; 14:1276951. https://doi.org/10.3389/fmicb.2023.1276951 DOI: https://doi.org/10.3389/fmicb.2023.1276951
12 Jia Z, Müller M, Le Gall T, Riool M, Müller M, Zaat SA, et al. Multiplexed detection and differentiation of bacterial enzymes and bacteria by color-encoded sensor hydrogels. Bioact Mater. 2021;6(12):4286. https://doi.org/10.1016/j.bioactmat.2021.04.022 DOI: https://doi.org/10.1016/j.bioactmat.2021.04.022
13.Wang J, Liang S, Lu X, et al. Bacteriophage endolysin Ply113 as a potent antibacterial agent against polymicrobial biofilms formed by enterococci and Staphylococcus aureus. Frontiers in Microbiology. 2023; 14:1304932.
https://doi.org/10.3389/fmicb.2023.1304932 DOI: https://doi.org/10.3389/fmicb.2023.1304932
14.Hao Y, Li L, Du W, Lu J. Shifting of Distribution and Changing of Antibiotic Resistance in Gram-Positive Bacteria from Bile of Patients with Acute Cholangitis. Infection and Drug Resistance. 2025; 18:1187-97. https://doi.org/10.2147/IDR.S482375 DOI: https://doi.org/10.2147/IDR.S482375
15.Yang S, Meng X, Zhen Y, et al. Strategies and mechanisms targeting Enterococcus faecalis biofilms associated with endodontic infections: a comprehensive review. Frontiers in Cellular and Infection Microbiology. 2024; 14:1433313. https://doi.org/10.3389/fcimb.2024.1433313 DOI: https://doi.org/10.3389/fcimb.2024.1433313
16.Abbas SN, Alwan TS, Hassan SM. Correlation between Diabetes Mellitus and Dyslipidemia Incidence in Center of Iraq. Azerbaijan Pharmaceutical and Pharmacotherapy Journal. 2023;22(1):38-40. https://doi.org/10.61336/appj/22-1-09 DOI: https://doi.org/10.61336/appj/22-1-09
17.Hussein GH, Mohammed RK. The Prevalence of Enterotoxin SEA and SEB genes in Staphylococcus aureus Multidrug-resistant Isolates from Clinical Specimens in Baghdad Province. Iraqi J Sci. 2024;65(11):6476-84. https://doi.org/10.24996/ijs.2024.65.11.24 DOI: https://doi.org/10.24996/ijs.2024.65.11.24
18.Najm AA, Flayyih MT. Detection of Endocarditis Associated Pili Genes in Enterococcus Faecalis Clinical Isolates. Ibn Al-Haitham J Pure Appl Sci. 2025;38(1):37-49. Available from: https://www.jih.uobaghdad.edu.iq/index.php/j/article/view/3539
19.TNVV, Premnath M, Stanley JV, et al. Whole genome sequencing based prediction of antimicrobial resistance evolution among the predominant bacterial pathogens of diabetic foot ulcer. World Journal of Microbiology & Biotechnology. 2025;41(5):161.
https://doi.org/10.1007/s11274-025-04362-2 DOI: https://doi.org/10.1007/s11274-025-04362-2
20.Noor Alhuda AK, Mahmood SS. The Prevalence of (Ompa, Csue) Genes Among Biofilm Producer Acinetobacter Baumannii Isolates. Iraqi Journal of Agricultural Sciences. 2024;55(5):1720-7. https://doi.org/10.36103/c5bw0g67 DOI: https://doi.org/10.36103/c5bw0g67
21.Evanson DJ, Elcic L, Uyeda JW, Zulfiqar M. Imaging of gallstones and complications. Current Problems in Diagnostic Radiology. 2025;54(3):392-403. https://doi.org/10.1067/j.cpradiol.2024.12.007 DOI: https://doi.org/10.1067/j.cpradiol.2024.12.007
22.Jha AK, Jha M, Adhikari S. Cholesterol Gallstone among Patients with Cholelithiasis Admitted to the Department of Surgery of a Tertiary Care Center. Journal of Nepal Medical Association. 2023;61(268):915-8.
https://doi.org/10.31729/jnma.8362 DOI: https://doi.org/10.31729/jnma.8362
23. Jones MW, Weir CB, Marietta M. Gallstones (cholelithiasis) [Internet]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025 Jan– [updated 2025 Jun 2; cited 2025]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459370/
24.Yuan S, Gill D, Giovannucci EL, Larsson SC. Obesity, Type 2 Diabetes, Lifestyle Factors, and Risk of Gallstone Disease: A Mendelian Randomization Investigation. Clinical Gastroenterology and Hepatology. 2022;20(3):e529-37. https://doi.org/10.1016/j.cgh.2020.12.034 DOI: https://doi.org/10.1016/j.cgh.2020.12.034
25.Xie L, Xu M, Lei Y, et al. The causal relationship between diet habits and cholelithiasis: a comprehensive Mendelian randomization (MR) study. Frontiers in Nutrition. 2024; 11:1377631. https://doi.org/10.3389/fnut.2024.1377631 DOI: https://doi.org/10.3389/fnut.2024.1377631
26.Zheng X, Yan Y, Li X, et al. Microbial characteristics of bile in gallstone patients: a comprehensive analysis of 9,939 cases. Frontiers in Microbiology. 2024; 15:1481112. https://doi.org/10.3389/fmicb.2024.1481112 DOI: https://doi.org/10.3389/fmicb.2024.1481112
27.Mustafa AH, Hameed AA. Bacteriological identification and prevalence of common microbiota involved in gallstone formation among Iraqi cholelithiasis patients. International Journal of Clinical Biology and Biochemistry. 2025;7(1):46-51.
https://doi.org/10.33545/26646188.2025.v7.i1a.83 DOI: https://doi.org/10.33545/26646188.2025.v7.i1a.83
28.Cozma MA, Găman MA, Diaconu CC, et al. Microbial Profile and Antibiotic Resistance Patterns in Bile Aspirates from Patients with Acute Cholangitis: A Multicenter International Study. Antibiotics. 2025;14(7):679. https://doi.org/10.3390/antibiotics14070679 DOI: https://doi.org/10.3390/antibiotics14070679
29.Zhang R, Chen C, Zheng S, et al. Preliminary study of biliary microbiota and identification of bacterial species associated with pigmented gallstone formation. Frontiers in Cellular and Infection Microbiology. 2025; 15:1532512.
https://doi.org/10.3389/fcimb.2025.1532512 DOI: https://doi.org/10.3389/fcimb.2025.1532512
30.Wang D, Ye A, Jiang N. The role of bacteria in gallstone formation. Folia Microbiologica. 2024;69(1):33-40.
https://doi.org/10.1007/s12223-024-01131-w DOI: https://doi.org/10.1007/s12223-024-01131-w
31.Wu X, Wang H, Xiong J, et al. Staphylococcus aureus biofilm: Formulation, regulatory, and emerging natural products-derived therapeutics. Biofilm. 2024; 7:100175. https://doi.org/10.1016/j.bioflm.2023.100175 DOI: https://doi.org/10.1016/j.bioflm.2023.100175
32.Tamrat E, Asmare Z, Geteneh A, et al. The global prevalence of biofilm-forming Enterococcus faecalis in clinical isolates: a systematic review and meta-analysis. BMC Infectious Diseases. 2025; 25:981. https://doi.org/10.1186/s12879-025-11399-z DOI: https://doi.org/10.1186/s12879-025-11399-z
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Ahmed M. Naji, Sameer A. Alash

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


