The Role of Arginase and some Parameters in Diabetic Type 2 Patients with and without Retinopathy
DOI:
https://doi.org/10.32007/jfacmedbaghdad.6632283الكلمات المفتاحية:
Arginase1، Diabetic Retinopathy، Type 2 Diabetes mellitus، Oxidative Stress، Lipid Profileالملخص
Background: Oxidative stress plays a major role in the pathogenesis of diabetes mellitus by damaging cellular organelles and enzymes in blood such as arginase1, insulin and glutathione s-transferase; increasing lipid peroxidation such as malondialdehyde and increasing insulin resistance which can lead to diabetic complications such as diabetic retinopathy.
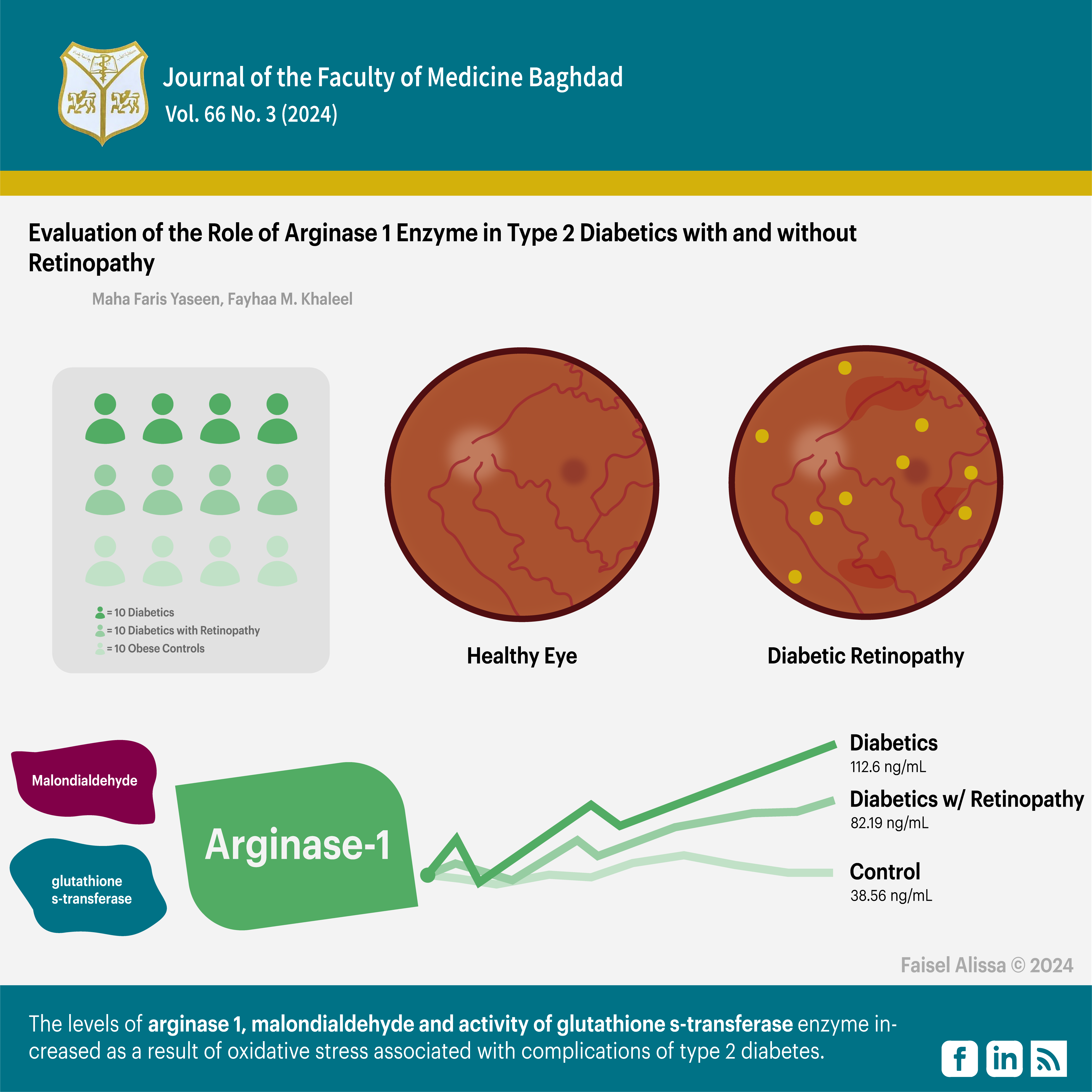
Objectives: To explore the relationship of oxidative stress to the development of diabetic retinopathy by measuring the levels of Arginase1, the activity of glutathione s-transferase enzyme, and the levels of malondialdehyde as a secondary product of lipid peroxidation (biomarker for oxidative stress).
Patients and MethodsThis study was conducted from November 2022 to January 2023 at the Ibn Al-Haitham Teaching Eye Hospital in Baghdad, the University of Baghdad / Department of Chemistry and the National Diabetes Centre for Treatment and Research at Al-Mustansyrriah University. This study was conducted on 120 subjects distributed as follows: 40 non-diabetic obese controls, 40 type 2 diabetics with no retinopathy, and 40 type 2 diabetic retinopathy patients, between 30 and 65 years of age. All groups were subjected to tests: measuring fasting blood glucose (FBG), HbA1c, lipid profile (cholesterol, triglycerides, HDL- cholesterol, LDL- cholesterol, and VLDL cholesterol), serum total arginase1, malondialdehyde glutathione s -transferase, body mass index (BMI), and waist-to-hip ratio.
Results: Mean arginase1 levels were significantly higher in diabetic patients than in diabetic retinopathy and control groups (p ≤ 0.05). The mean xidative stress marker malondialdehyde concentration was significantly higher in diabetic retinopathy patients than in type 2 diabetics and the control group (P ≤ 0.05). The mean glutathione s-transferase activity in diabetic retinopathy patients was significantly higher than in the control group and type 2 diabetics (P ≤ 0.05).
Conclusion: There is a relationship between oxidative stress and the development of diabetic retinopathy, where the levels of arginase1 and malondialdehyde increased and the activity of glutathione s –transferase enzyme increased as a result of oxidative stress and inflammation associated with complications of type 2 diabetes.
التنزيلات
المراجع
Jaid HK, Khaleel FM, Salman IN, Abd BA. Estimation of Apelin Levels in Iraqi Patients with Type II Diabetic Peripheral Neuropathy. Baghdad Sci. J. 2023;20(5):1684. https://doi.org/10.21123/bsj.2023.7566.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat. Rev. Endocrinol. 2020;16(7):349-62. https://doi.org/10.1038/s41574-020-0355-7.
Verhulst MJ, Loos BG, Gerdes VE, Teeuw WJ. Evaluating all potential oral complications of diabetes mellitus. fendo. 2019;10:56. https://doi.org/10.3389/fendo.2019.00056.
Fahmy R, Almutairi NM, Al-Muammar MN, Bhat RS, Moubayed N, El-Ansary A. Controlled diabetes amends oxidative stress as mechanism related to severity of diabetic retinopathy. Sci. Rep. 2021; 11(1):17670. https://doi.org/10.1038/s41598-021-96891-7.
Shosha E, Fouda AY, Narayanan SP, Caldwell RW, Caldwell RB. Is the arginase pathway a novel therapeutic avenue for diabetic retinopathy? J. Clin. Med. 2020;9(2):425. https://doi.org/10.3390/jcm9020425.
Augustine J, Troendle EP, Barabas P, McAleese CA, Friedel T, Stitt AW, et al. The role of lipoxidation in the pathogenesis of diabetic retinopathy. fendo. 2021 Feb 18;11:621938. https://doi.org/10.3389/fendo.2020.621938
Shawki HA, Elzehery R, Shahin M, Abo-Hashem EM, Youssef MM. Evaluation of some oxidative markers in diabetes and diabetic retinopathy, Diabetol. Int. 2021; 12: 108-17. https://doi.org/10.1007/s13340-020-00450-w.
Khan M, Nouman M, Hashim H, et.al. A correlation biomarker between BMI and lipid peroxidation in type 2 diabetes mellitus with and without other complications. Biol. Clin. Sci. Res. 2023 ;(1) https://doi.org/10.54112/bcsrj.v2023i1.253.
Ali SE, Khaleel FM. Assessing the Activity of Renin and GST in the Serum of Ladies Suffering from Polycystic Ovary Syndrome and COVID-19 to Predict the Danger of Cardiac Disease. Baghdad Sci. J. 2023;20(3 (Suppl.)):0986-.https://doi.org/10.21123/bsj.2023.7879.
Ren Y, Li Z, Li W, Fan X, Han F, Huang Y, et al. Arginase: Biological and Therapeutic Implications in Diabetes Mellitus and Its Complications. Oxid. Med. Cell. Longev. 2022; 26. https://doi.org/10.1155/2022/2419412
Khaleel F, N-Oda N, Abed BA. Disturbance of Arginase Activity and Nitric Oxide Levels in Iraqi Type 2 Diabetes Mellitus. Baghdad Sci. J 2018;15(2). https://doi.org/10.21123/bsj.2018.15.2.0189
Kövamees O, Shemyakin A, Checa A, Wheelock CE, Lundberg JO, Östenson CG, et al. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J.Clin.Endocrinol Metabo. 2016; 101(11):3952-8. https://doi.org/10.1210/jc.2016-2007
Ekinci D, Cankaya M, Gül İ, Coban TA. Susceptibility of cord blood antioxidant enzymes glutathione reductase, glutathione peroxidase and glutathione S-transferase to different antibiotics: in vitro approach. J. Enzyme Inhib. Chem. 2013; 28(4):824-9. https://doi.org/10.3109/14756366.2012.688042.
Abbasnasab Sardareh S, Brown GT, Denny P. Comparing four contemporary statistical software tools for introductory data science and statistics in the social sciences. Teaching Statistics. 2021; 43: S157-72. https://doi.org/10.1111/test.12274
Anjana RM, Baskar V, Nair AT, Jebarani S, Siddiqui MK, Pradeepa R, Unnikrishnan R, Palmer C, Pearson E, Mohan V. Novel subgroups of type 2 diabetes and their association with microvascular outcomes in an Asian Indian population: a data-driven cluster analysis: the INSPIRED study. BMJ Open Diabetes Research and Care. 2020; 8(1): e001506. http://dx.doi.org/10.1136/bmjdrc-2020-001506
Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes?. Cell metabolism. 2022; 34(1):11-20. https://doi.org/10.1016/j.cmet.2021.12.012
Almutairi NM, Alahmadi S, Alharbi M, Gotah S, Alharbi M. The association between HbA1c and other biomarkers with the prevalence and severity of diabetic retinopathy. Cureus. 2021; 13(1). https://doi.org/10.7759/cureus.12520.
Shahid SU, Sarwar S. The abnormal lipid profile in obesity and coronary heart disease (CHD) in Pakistani subjects. LIPIDS HEALTH DIS 2020; 19(1):1-7. https://doi.org/10.1186/s12944-020-01248-0.
Shosha E, Fouda AY, Narayanan SP, Caldwell RW, Caldwell RB. Is the arginase pathway a novel therapeutic avenue for diabetic retinopathy? J. clin. Med. 2020; 9(2):425. https://doi.org/10.3390/jcm9020425
Hussain HA, Muftin NQ, Al-Jibouri MN, Salah GB. Study of the Arginase Activity and Other Biochemical Parameters in Patients with Coronary Artery Disease in Baghdad Governorate-Iraq. (MJS). 2023; 34(1):37-44. http://doi.org/10.23851/mjs.v34i1.1251
Mengozzi A, Costantino S, Paneni F, Duranti E, Nannipieri M, Mancini R, et al. Targeting SIRT1 rescues age-and obesity-induced microvascular dysfunction in ex vivo human vessels. Circulation Research. 2022 Sep 2; 131(6):476-91. https://doi.org/10.1161/CIRCRESAHA.122.320888
Paha S, Sharma R, Singh B. Role of glutathione S-transferase in coronary artery disease patients with and without type 2 diabetes mellitus. J Clin Diagn Res: JCDR. 2017; 11(1):BC05. https://doi.org/10.7860/JCDR/2017/23846.9281.
Merkhan M, Mohammad J, Fathi Z, Younus Z, Mahmood SM, Mohammed M. Silent hyperlipidaemia modulated vascular endothelial markers. Pharmacia 68 (2): 479–484. https://doi.org/10.3897/pharmacia.68.e67959
Khalili F, Vaisi‐Raygani A, Shakiba E, Kohsari M, Dehbani M, Naseri R, et al. Oxidative stress parameters and keap 1 variants in T2DM: Association with T2DM, diabetic neuropathy, diabetic retinopathy, and obesity. J.Clin.Lab.Anal. 2022;36(1):e24163. https://doi.org/10.1002/jcla.24163.
Masi S, Colucci R, Duranti E, Nannipieri M, Anselmino M, Ippolito C, et al. Aging modulates the influence of arginase on endothelial dysfunction in obesity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018; 38(10):2474-83. https://doi.org/10.1161/ATVBAHA.118.311074.
Al-Anbari AA, Alta'ee AH, Al-Saad SF. A study of arginase-1 activity and lipid profile in patients with myocardial infarction. J Adv Biotechnol Exp Ther. 2022; 5(3):553-61. https://doi.org/10.5455/jabet.2022.d135.
Mazrouei S, Petry SF, Sharifpanah F, Javanmard SH, Kelishadi R, Schulze PC, et al. Pathophysiological correlation of arginase-1 in development of type 2 diabetes from obesity in adolescents. (BBA). 2023; 1867(2):130263.https://doi.org/10.1016/j.bbagen.2022.130263.
Gunawardena HP, Silva KD, Sivakanesan R, Katulanda P. Increased lipid peroxidation and erythrocyte glutathione peroxidase activity of patients with type 2 diabetes mellitus: Implications for obesity and central obesity. Obesity Medicine. 2019; 15:100118. https://doi.org/10.1016/j.obmed.2019.100118.
Varbo A, Freiberg JJ, Nordestgaard BG. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the Copenhagen General Population Study. Clin. Chem. 2018; 64(1):219-30. https://doi.org/10.1373/clinchem.2017.279463
An H, Du X, Huang X, Qi L, Jia Q, Yin G, et al. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl. psychiatry. 2018; 8(1):258. https://doi.org/10.1038/s41398-018-0303-7.
Jiang F, Zhou L, Zhang C, Jiang H, Xu Z. Malondialdehyde levels in diabetic retinopathy patients: a systematic review and meta-analysis. Chin. Med. J. 2023; 136(11):1311-21. https://doi.org/10.1097/CM9.0000000000002620.
Caldwell RW, Caldwell RB. Is the Arginase Pathway a Novel Therapeutic Avenue for Diabetic Retinopathy?. J. Clin. Med. 2020; 9:425. https://doi.org/10.3390/jcm9020425.
Sharif S, Maqsood M, Naz S, Manzoor F, Farasat T. Expression of GSTT1 in type 2 diabetic retinopathy patients. CRIT REV EUKAR GENE Expression. 2019; 29(1). https://doi.org/10.1615/CritRevEukaryotGeneExpr.2019025074.
Zhong Y, Yue S, Wu J, Guan P, Zhang G, Liu L, et al. Association of the serum total cholesterol to triglyceride ratio with diabetic retinopathy in Chinese patients with type 2 diabetes: a community-based study. Diabetes Therapy. 2019; 10: 597-604. https://doi.org/10.1007/s13300-019-0579-5
Ali SE, Khaleel FM, Ali FE. A Study of Apelin-36 and GST Levels with Their Relationship to Lipid and Other Biochemical Parameters in the Prediction of Heart Diseases in PCOS Women Patients. Baghdad Sci. J. 2020; 17(3(Suppl.):0924. http://dx.doi.org/10.21123/bsj.2020.17.
Femlak M, Gluba-Brzózka A, Ciałkowska-Rysz A, Rysz J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids in health and disease. 2017; 16(1):1-9. https://doi.org/10.1186/s12944-017-0594-3.
Namitha D, Nusrath A, Rani NA, Dhananjaya SY, Lokanathan TH, Kruthi BN, et al. Role of lipid indices in the assessment of microvascular risk in type 2 diabetic retinopathy patients. Cureus. 2022;14(3). https://doi.org/10.7759/cureus.23395.
التنزيلات
منشور
إصدار
القسم
الرخصة
الحقوق الفكرية (c) 2024 Maha F. Yassen, Fayhaa. M. Khaleel

هذا العمل مرخص بموجب Creative Commons Attribution 4.0 International License.




















 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


